0310
Dynamic 13C MRS using 13C/1H Multichannel Coil as an Alternative to Imaging for Hyperpolarized Pyruvate Brain Studies1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 2Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 3GE Healthcare, Dallas, TX, United States, 4Biochemistry and Chemical Biology, UT Dallas, Richardson, TX, United States, 5Internal Medicine, UT Southwestern Medical Center, Dallas, TX, United States, 6Neurological Surgery, UT Southwestern Medical Center, Dallas, TX, United States, 7Electrical and Computer Engineering, UT Dallas, Richardson, TX, United States
Synopsis
This study demonstrates that time-resolved 13C MR spectroscopy with the multichannel 13C/1H RF coils can be performed as an alternative to imaging for assessing pyruvate metabolism using hyperpolarized [1-13C]pyruvate in the human brain.
Background
Recent advances in clinical translation of hyperpolarized [1-13C]pyruvate imaging1 has enabled metabolic imaging of the human brain in healthy subjects2,3 and patients with brain tumors4–6 or traumatic brain injury.7 However, accurate and reliable imaging of pyruvate metabolism in the brain is still challenging, especially for the measurement of [13C]bicarbonate production, which is a salient surrogate biomarker of in vivo pyruvate dehydrogenase activity and, potentially, mitochondrial integrity. Previous studies reported inconsistent measurement of [13C]bicarbonate, ranging from less than 2%8 to 8%3 relative to the [1-13C]pyruvate signals. In this study, we propose to use small flip-angle time-resolved 13C MRS with a multichannel 13C/1H coil as an alternative to imaging to achieve reliable measurements and analysis of hyperpolarized [1-13C]pyruvate and its products, [1-13C]lactate and [13C]bicarbonate, in the brain from healthy volunteers.Methods
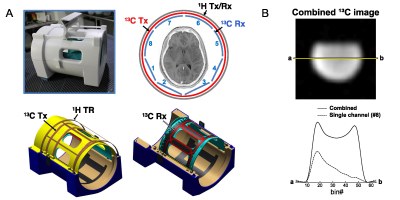
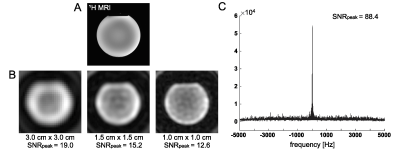
All MR studies were performed using a wide-bore 3T 750w MR scanner (GE Healthcare, Waukesha, Wisconsin, USA). A 13C/1H dual-frequency RF head coil that consists of 1H quadrature transmit (Tx) and receive (Rx), 13C quadrature Tx and 8-channel 13C Rx arrays (Clinical MR Solutions, USA) was developed for the study.9 The structure of the head coil is shown in Figure 1A. Rx performance was tested using a cylindrical phantom (diameter = 14 cm) that contains non-labeled pure ethylene glycol (natural abundance 1.1% of 13C), Figure 1B. At the center of the cylindrical phantom, the coil sensitivity was 78% of the phantom. The cross-sectional signal changes demonstrate the reliable and relatively homogeneous signal detection from the entire phantom when all the coil elements are combined, and region-focused detections from individual coils.To demonstrate the SNR advantage of a small flip-angle MRS over MRI, a phantom test was performed using the spherical [13C]bicarbonate phantom. A slice-selective single-shot spiral 13C imaging (flip-angle = 90°, slice thickness = 15 mm, TR = 3 s, FOV = 24 cm × 24 cm, 128 averages) was applied to acquire 2D 13C images with three different nominal in-plane spatial resolutions (3.0 cm × 3.0 cm, 1.5 cm × 1.5 cm, 1.0 cm × 1.0 cm) by adjusting the spiral readouts. With similar settings, 13C free induction decay (FID) was also acquired using a 6.5° slice-selective excitation (spectral bandwidth = 10000 Hz, spectral points = 4096).
Four healthy adults were recruited for the study (age: 24 – 65 years, 1 male and 3 females, body mass index = 26.4 ± 2.9). Each participant was imaged with the brain MR protocol, which includes two injections of hyperpolarized [1-13C]pyruvate and a series of 1H MRI. The imaging protocol was approved by the local institutional review board (IRB#: STU 072017-009, ClinicalTrials.gov ID: NCT03502967). A clinical SPINlabTM polarizer (GE Healthcare) was used for the dynamic nuclear polarization (DNP), and pyruvate samples were polarized as previously described.10
All the in vivo 13C data were reconstructed in absorption mode via a custom-made reconstruction using MATLAB (MathWorks, MA), and metabolite peaks were quantified from time-averaged 13C spectra.11 Brain extraction and segmentation for 1H human brain image was conducted using FSL package (FMRIB, UK).12,13 Data are presented as mean ± standard deviation. Statistical significance in metabolite ratios between brain regions was evaluated by a paired t-test (α = 0.05, two-tailed). Least square fitting was used for linear regression between brain volumes and hyperpolarized signals, and Pearson product-moment correlation coefficient (r) was calculated to measure the linear relationship.
Results and Discussion
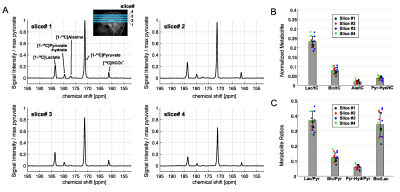
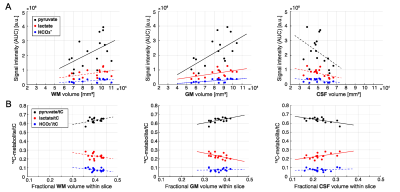
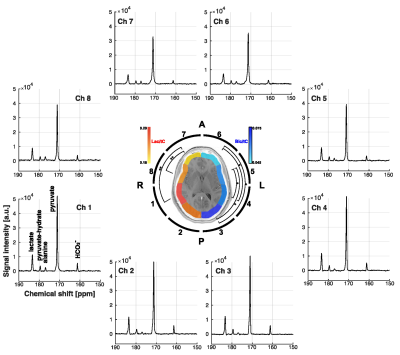
In the phantom study, shown in Figure 2, SNRpeak from the MRS spectrum was 4.6×, 5.8× and 7.0× higher than SNRpeak of 13C images with 3 cm, 1.5 cm, and 1 cm of nominal spatial resolution, respectively. Time-averaged spectra of slice #1 – 4 from a representative participant are shown in Figure 3A. [1-13C]Lactate relative to the total hyperpolarized 13C signal (tC) was consistent throughout the slices, ranging from 0.212 to 0.244. [13C]bicarbonate production was 30 – 37 % of lactate (Fig. 3B-C). Areas under the curves (AUCs) for [1-13C]pyruvate, [1-13C]lactate, and [13C]bicarbonate signals were positively correlated to the volume of grey matter (GM, r > 0.5, p ≤ 0.03) and white matter (WM, r > 0.4, p = 0.06 – 0.10), and negatively correlated to cerebrospinal fluid (CSF, r < -0.4) volumes, respectively, Figure 4A. The pyruvate signal normalized by tC maintained the positive correlation with fractional GM (r = 0.57, p = 0.02) volumes and negative correlation with fractional CSF volume (r = -0.64, p = 0.008), Figure 4B. However, lactate/tC showed a strong negative correlation with fractional GM volume (r = -0.54, p = 0.03) and a positive correlation with fractional CSF volume (r = 0.54, p = 0.03). Region-weighted assessment, as shown in Figure 5, demonstrated that region-weighted [1-13C]lactate/tC and [13C]bicarbonate/tC could be generated from coil-wise assessment of the metabolites in the time-averaged spectra.Conclusion
Dynamic MRS in combination with the 13C/1H multichannel RF coil is an affordable and reliable alternative to imaging methods in investigating cerebral metabolism using hyperpolarized [1-13C]pyruvate.Acknowledgements
Personnel Support: We appreciate the clinical research team and the supporting staffs of the Advanced Imaging Research Center at UT Southwestern for recruiting and imaging the volunteers – Jeannie Baxter, Lucy Christie, Kelley Derner, Maida Tai and Salvador Pena. We thank Ralph S. Hashoian for building the RF head coil.
Funding: The Mobility Foundation; The Texas Institute for Brain Injury and Repair; National Institutes of Health of the United States (R01 NS107409, P41 EB015908, S10 OD018468, S10 RR029119); The Welch Foundation (I-2009-20190330); UT Dallas Collaborative Biomedical Research Award (UTD 1907789).
References
1. Nelson SJ, Kurhanewicz J, Vigneron DB, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci Transl Med. 2013;5:198ra108.
2. Lee CY, Soliman H, Geraghty BJ, et al. Lactate topography of the human brain using hyperpolarized 13C-MRI. NeuroImage. 2020;204:116202.
3. Grist JT, McLean MA, Riemer F, et al. Quantifying normal human brain metabolism using hyperpolarized [1-13C]pyruvate and magnetic resonance imaging. NeuroImage. 2019;189:171–179.
4. Chen J, Patel TR, Pinho MC, et al. Preoperative imaging of glioblastoma patients using hyperpolarized 13C pyruvate: Potential role in clinical decision making. Neuro Oncol Adv. 2021;3(1):vdab092.
5. Miloushev VZ, Granlund KL, Boltyanskiy R, et al. Metabolic imaging of the human brain with hyperpolarized 13C pyruvate demonstrates 13C lactate production in brain tumor patients. Cancer Res. 2018;78(14):3755–3760.
6. Park I, Larson PEZ, Gordon JW, et al. Development of methods and feasibility of using hyperpolarized carbon-13 imaging data for evaluating brain metabolism in patient studies. Magn Reson Med. 2018;80(3):864–873.
7. Hackett EP, Pinho MC, Harrison CE, et al. Imaging acute metabolic changes in patients with mild traumatic brain injury using hyperpolarized [1-13C]pyruvate. iScience. 2020;23(12):101885.
8. Gordon JW, Chen H-Y, Autry A, et al. Translation of carbon-13 EPI for hyperpolarized MR molecular imaging of prostate and brain cancer patients. Magn Reson Med. 2019;81(4):2702–2709.
9. Ma J, Hashoian R, Sun C, et al. Development of 1H/13C RF head coil for hyperpolarized 13C imaging of human brain. Proceedings of the 27th ISMRM, Montreal, Canada. 2019;#568.
10. Ma J, Chen J, Reed GD, et al. Cardiac T2∗ measurement of hyperpolarized 13C metabolites using metabolite-selective multi-echo spiral imaging. Magn Reson Med. 2021;86(3):1494-1504.
11. Ma J, Pinho MC, Harrison CE, et al. Dynamic 13C MR spectroscopy as an alternative to imaging for assessing cerebral metabolism using hyperpolarized pyruvate in humans. Magn Reson Med. 2021. In press. doi:10.1002/mrm.29042 .
12. Smith SM. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155.
13. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20(1):45–57.
Figures