0297
Super-fast and accurate estimation of relaxation and chemical exchange rate using 31P-MT-MR fingerprinting at 7T in the human brain1CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 2Laboratory for Functional and Metabolic Imaging, EPFL, Lausanne, Switzerland, 3Animal Imaging and Technology, EPFL, Lausanne, Switzerland
Synopsis
In this abstract, we introduce 31P-MT-MRF, using a SAR efficient magnetization transfer (MT) approach. We extended the magnet resonance fingerprinting (MRF) framework to overcome obstacles of in vivo human brain 31P measurements. This abstract reports the reproducibility and robustness of 31P-MT-MRF estimations of the relaxation parameters of y-ATP and PCr as well as the chemical exchange rate kCK on healthy volunteers in vivo in human brain at 7T. We were able to demonstrate that our method is 3 times faster as state-of-the-art MT methods [6] and estimates are bias free enabling ultra-fast kCK measurement in 2:15 min scan time.
Introduction
ATP is known to be fundamental for supporting various cellular activities in human brain. A stable ATP concentration is maintained by phosphocreatine (PCr) acting as a reservoir and carrier in the creatine kinase (CK) cycle. This chemical exchange rate kCK is usually studied in the human brain by using 31P-MRS combined with magnetization transfer (MT) or saturation transfer (ST) experiments [3-7]. However, these methods are time consuming, due to the long relaxation time of PCr and the low signal sensitivity in 31P-MRS. Recently a novel multi-parametric estimation framework for MRI was introduced, called magnetic resonance fingerprinting [1]. Using a fast-steady state free precision type sequence enables a time efficient and robust acquisition even for low signals. In a preclinical study [2] it was shown that kCK can be estimated using saturation transfer MRF approach with a two-pool model. However, a long saturation pulse train leads to excessive RF heat deposition that limiting its translation to a clinical scanner.In this abstract, we introduce 31P-MT-MRF, using a SAR efficient MT approach. We extended the framework to overcome obstacles of in vivo human brain 31P measurements. This abstract reports the reproducibility and robustness of 31P-MT-MRF estimations of the relaxation parameters of y-ATP and PCr as well as the chemical exchange rate kCK on healthy volunteers in vivo in human brain at 7T. We were able to show that our method is 3 times faster as state-of-the-art MT methods [6] and estimates are bias free enabling ultra-fast kCK measurement in 2:15 min scan time.
Method
The MRS-FP approach is a b-SSFP like scheme with fast repeating slice selective 1.5 ms Shinar-le-Roux Pulses to excite a 20mm slice in the occipital lobe (Figure 1). The TR is set constant to 18.9ms with a 16.67ms acquisition time. The scheme consists out of 3 parts. Part one starts with a global inversion pulse followed by 699 RF excitations. The sinusoidal shapes in the first half showed to be sensitive for T1 and T2 changes (relaxation part), whereas the second fast changing part is mostly sensitive to γB1+ inhomogeneities and off resonances (inhomogeneity part) [8]. This scheme was repeated n times with 6 sec time gap in between. The third part to increase kCK sensitivity with 244 FAs in sinusoidal pattern follows afterwards and is repeated n times with 4 sec relaxation time. In each repetition, Pi and PCr are inverted asymmetrically using an adiabatic inversion pulse [10] as preparation. After 185 FAs and a short 200 ms relaxation time a second asymmetric inversion pulse with opposite pulse profile to invert α, β, γ-ATP is used. A Gradient echo B0 map (30mm slice; 6 s) was acquired afterwards to incorporate B0 inhomogeneities into the dictionary.The Results where compared with the EBIT MT approach [6], adapted with 1D localization using the same type of excitation pulse as in the MRF scheme. 38averages and 1 dummy scan were acquired for MRF and 16+1 scans for the EBIT, resulting in a similar scan time of 18.5 min. In vivo data was acquired from 6 healthy subject (2 female; 4 males; age 19-27 years), who provided written informed consent. To prove the reproducibility of our method the subjects were scanned 2 times with the same MRF and EBIT protocol, interrupted by a 15 min break outside of the MRI scanner. All MR experiments were performed on a 7T/68cm MR scanner (Siemens Medical Solutions, Erlangen, Germany) with a 1H quadrature surface coil (10cm-diameter) and a single-loop 31P coil (7cm-diameter) for the occipital lobe.
A dictionary was created for each subject using the Bloch-McConnel equations and a two-pool model [2]. Slice profile correction [9], B0 distribution and a linear coil sensitivity approximation of the surface coil were incorporated in the simulation using isochromats. T2,γATP was set to 30ms and the resonance frequency offset fγATP to 304 Hz, resulting in 7 remaining parameters to fit with γB1+, the mean off-resonance foff, T1,PCr, T1,γATP, T2,PCr, M0PCr and kCK.
Results
Table 1 shows the estimated mean values of T1,PCr, T1,γATP, T2,PCr, M0PCr and kCK compared to the state of the art EBIT estimation. The mean estimation over all volunteers and scans are in good range to each other. Table 2 is summarizing the mean and standard deviation (STD) for different number of estimations. With decreasing number of averages, the STD is increasing, however, the estimations stay biasfree. Figure 2 shows this benefit, compared to the biasing estimations of EBIT. The mean test-retest reproducibility is validated in Figure 3 using the coefficient of variation (CV). For a scan time of 6 min, the CV of kCK is still below the CV of EBIT with 18.5 min scan time.Discussion
This study shows that with 31P-MT-MRF T1,PCr, T1,γATP, T2,PCr, M0PCr and kCK can be estimated fast and accurately. A scan time reduction of factor 3 can be achieved with the proposed scheme. Additional γB1+, foff, T2,PCr and M0PCr are estimated. Due to bias free estimation the scan time can be further reduced in cost of higher STD. With a 2.15 min scan time the CV stays below 20%, which outperforms ST methods [7]. Further scheme optimizations can improve this method.Acknowledgements
This work was supported by the Swiss National Science Foundation (grants n° 320030_189064). We acknowledge access to the facilities and expertise of the CIBM Center for Biomedical Imaging, a Swiss research center of excellence founded and supported by Lausanne University Hospital (CHUV), University of Lausanne (UNIL), Ecole polytechnique fédérale de Lausanne (EPFL), University of Geneva (UNIGE) and Geneva University Hospitals (HUG).
References
[1] Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature 2013;495:187–192 doi: 10.1038/nature11971.
[2] Wang CY, Liu Y, Huang S, Griswold MA, Seiberlich N, Yu X. 31 P magnetic resonance fingerprinting for rapid quantification of creatine kinase reaction rate in vivo. NMR Biomed. 2017;30:1–14 doi: 10.1002/nbm.3786.
[3] Lei H, Zhu XH, Zhang XL, Ugurbil K, Chen W. In vivo 31P magnetic resonance spectroscopy of human brain at 7 T: An initial experience. Magn. Reson. Med. 2003;49:199–205 doi: 10.1002/mrm.10379.
[4] Du F, Zhu XH, Qiao H, Zhang X, Chen W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn. Reson. Med. 2007;57:103–114 doi: 10.1002/mrm.21107.
[5] Du F, Cooper AJ, Thida T, Sehovic S, Lukas SE, Cohen BM, Zhang X, Ongür D. In vivo evidence for cerebral bioenergetic abnormalities in schizophrenia measured using 31P magnetization transfer spectroscopy. JAMA Psychiatry. 2014 Jan;71(1):19-27. doi: 10.1001/jamapsychiatry.2013.2287.
[6] Ren, Jimin, A. Dean Sherry, and Craig R. Malloy. "Efficient 31P band inversion transfer approach for measuring creatine kinase activity, ATP synthesis, and molecular dynamics in the human brain at 7 T." Magnetic resonance in medicine 78.5 (2017): 1657-1666.
[7] Xin, Lijing, et al. "Nutritional ketosis increases NAD+/NADH ratio in healthy human brain: an in vivo study by 31P-MRS." Frontiers in nutrition 5 (2018): 62.
[8] Buonincontri, Guido, et al. "Spiral MR fingerprinting at 7 T with simultaneous B1 estimation." Magnetic resonance imaging 41 (2017): 1-6.
[9] Ma, Dan, et al. "Slice profile and B1 corrections in 2D magnetic resonance fingerprinting." Magnetic resonance in medicine 78.5 (2017): 1781-1789.
[10] Hwang, Tsang-Lin, Peter CM Van Zijl, and Michael Garwood. "Asymmetric adiabatic pulses for NH selection." (1999): 173-177.
Figures
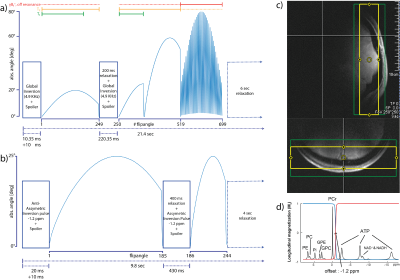
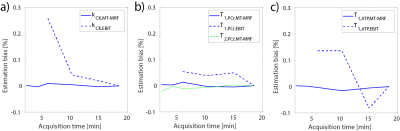
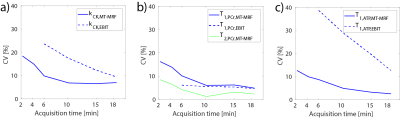
Figure 3: Mean CV [%] over the acquisition time for (a) kCK, (b) T1,PCr & T2,PCr and (c) T1,γATP comparing MT-MRF with EBIT.