0284
Hyperpolarized [1-13C]-alanine monitors tumor immortality and detects response to therapy in patient-derived glioblastoma models in vivo1Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Neurological Surgery, University of California San Francisco, San Francisco, CA, United States
Synopsis
Telomerase reverse transcriptase (TERT) expression is essential for tumor proliferation. TERT also reprograms metabolism by elevating NADH and upregulating the alanine transporter ASCT2. Here, we exploit these TERT-associated metabolic alterations for non-invasive imaging in live GBM cells and orthotopic tumors using hyperpolarized [1-13C]-alanine. Combined treatment with the TERT inhibitor 6-thio-2’-deoxyguanosine and the ASCT2 inhibitor V-9302 inhibits GBM proliferation, identifying a novel therapeutic opportunity. Importantly, lactate production from hyperpolarized [1-13C]-alanine is an early biomarker of response to treatment in vivo, prior to the onset of anatomical alterations. Our findings pave the way for improved therapy and response assessment for GBM patients.
Introduction
Telomere shortening constitutes a barrier to uncontrolled proliferation1. All tumors must find a mechanism of maintaining telomere length and most, including high-grade glioblastomas (GBMs) achieve this via reactivation of telomerase reverse transcriptase (TERT) expression1,2. Since TERT is silenced in normal cells and drives tumor immortality, TERT is a biomarker of tumor proliferation1,2. TERT is also a therapeutic target and the TERT inhibitor 6-thio-2’-deoxyguanosine (6-thio-dG) is in cancer clinical trials3.Studies indicate that TERT elevates NADH and upregulates the alanine transporter ASCT2 in low-grade oligodendrogliomas, providing a rationale for the use of hyperpolarized [1-13C]-alanine to monitor TERT expression4. These studies also point to the potential therapeutic utility of ASCT2 inhibitors such V-93025 for GBMs. Therefore, the goal of this study was to determine whether 1) hyperpolarized [1-13C]-alanine reports on TERT expression in GBMs 2) a combination of 6-thio-dG and V-9302 inhibits GBM proliferation and 3) hyperpolarized [1-13C]-alanine provides a readout of treatment response in vivo.
Methods
Cell models: We studied patient-derived GBM models (GBM1 and GBM6) that use TERT for telomere maintenance. Cells were maintained as described previously6,7.Hyperpolarized alanine preparation: [1-13C]-alanine was prepared as described4, polarized for ~1.5h, and dissolved in isotonic buffer to a final concentration of 38.9mM (cell studies) or 195mM (in vivo studies).
Treatment: siRNAs against TERT or non-targeting siRNA pool were used to transiently silence gene expression in GBM1 and GBM6 cells4. Alternatively, cells were treated with vehicle control (saline), 6-thio-dG (10μM), V-9302 (10μM) or a combination (10μM each) for 72h. For in vivo studies, rats bearing orthotopic GBM6 tumors were intraperitoneally treated with a combination of 6-thio-dG and V-9302 (50mg/kg each) daily.
Hyperpolarized 13C-MRS in live cells: Hyperpolarized [1-13C]-alanine was injected into ~3x107 live cells in a 5mm tube and 13C-MR spectra acquired every 3s for 300s on a Varian 500MHz spectrometer using a 13° pulse. Signal-to-noise (SNR) ratios were quantified in summed spectra using MestReNova.
Hyperpolarized 13C-MRS in vivo: MR studies were performed on a horizontal Bruker 3T scanner equipped with a quadrature 1H-13C volume coil. Athymic nu/nu rats were intracranially injected with 3x105 GBM6 cells4,8. Tumor growth was assessed by T2-weighted MRI using a spin echo (TurboRARE) sequence4,8. Following intravenous injection of 2.2ml of hyperpolarized [1-13C]-alanine, 2D echo planar spectroscopic imaging (EPSI) data was acquired with a spatial resolution of 5.375x5.375x8mm3 (TR=3s)4,8. 13C spectra were analyzed by calculating the area under alanine and lactate peaks. Intensity heat maps were produced by interpolating the data using a Lanczos-2 kernel. Ratios of lactate/alanine SNR were evaluated in Matlab4,8.
Statistical analysis: All results are expressed as mean±STD. Statistical significance was assessed using an unpaired two-tailed Student’s t-test with p<0.05 considered significant.
Results and Discussion
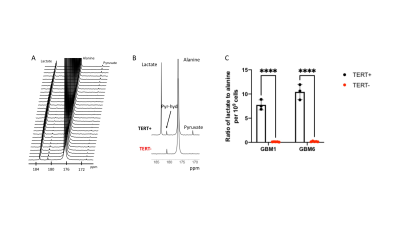
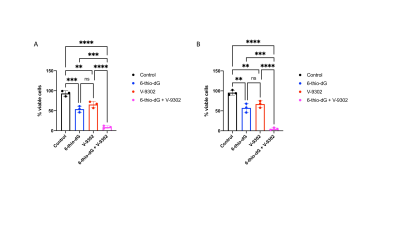
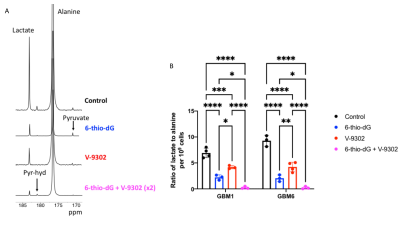
Lactate production from hyperpolarized [1-13C]-alanine is a biomarker of TERT expression in GBM cells: Fig 1A-1B show a representative 13C spectral array and summed 13C spectra from GBM1 TERT+ and TERT- cells. Importantly, lactate production from hyperpolarized [1-13C]-alanine was significantly reduced in TERT- cells relative to TERT+ in both GBM1 (98.6% drop, p=0.006; Fig.1C) and GBM6 (98.3% drop, p=0.008; Fig.1C) models.Treatment with a combination of 6-thio-dG and V-9302 inhibits GBM proliferation, an effect that can be detected by hyperpolarized [1-13C]-alanine: Next, we examined the effect of treatment with 6-thio-dG, V-9302 or a combination of 6-thio-dG and V-9302 on GBM1 and GBM6 cells. As shown in Fig. 2A-2B, while both compounds individually inhibited proliferation, the combination of 6-thio-dG and V-9302 maximally inhibited cell viability. Importantly, the reduction in lactate production from hyperpolarized [1-13C]-alanine paralleled the effect of these inhibitors on GBM growth. Representative 13C summed spectra from GBM6 cells treated with vehicle control (saline), V-9302, 6-thio-dG or the combination are shown in Fig. 3A. The combination of 6-thio-dG and V-9302 resulted in the highest inhibition of lactate production in both GBM1 and GBM6 models (Fig. 3B).
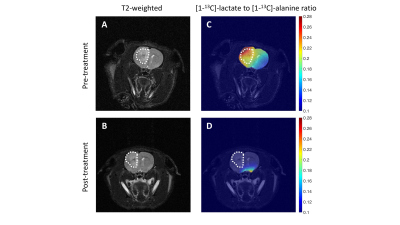
Hyperpolarized [1-13C]-alanine monitors response to therapy in vivo: Finally, we performed 2D EPSI studies in rats bearing orthotopic GBM6 tumors to determine the ability of hyperpolarized [1-13C]-alanine to assess response to the combination of 6-thio-dG and V-9302. Examination of metabolic heatmaps (Fig. 4A, 4C) confirmed that the lactate/alanine ratio was higher in tumor relative to contralateral normal brain, consistent with previous results in TERT+ oligodendrogliomas4. Importantly, lactate /alanine heatmaps generated 7 days after treatment with a combination of 6-thio-dG and V-9302 showed a drop relative to the pre-treatment heatmap (Fig. 4C-4D). There was no change in tumor volume until day 21 (Fig. 4A & 4B; pre-treatment=85mm3, post-treatment=80mm3; day 21=3mm3), suggesting that lactate production from hyperpolarized [1-13C]-alanine has the potential to serve as an early biomarker of GBM response to therapy, prior to anatomical alterations in vivo.
Conclusions
TERT expression drives tumor immortality, making TERT both a tumor biomarker and a therapeutic target1,2. Our studies with patient-derived GBM cells and orthotopic tumor xenografts suggest that lactate production from hyperpolarized [1-13C]-alanine is a non-invasive metabolic imaging biomarker of TERT expression in GBMs. Combined treatment with the TERT inhibitor 6-thio-dG and the ASCT2 inhibitor V-9302 abrogates GBM proliferation, identifying a novel therapeutic opportunity for GBMs. Importantly, lactate production from hyperpolarized [1-13C]-alanine serves as a companion imaging biomarker of GBM response to therapy, at early timepoints prior to volumetric alterations.Acknowledgements
We thank William Byrne for his support in the UCSF preclinical MR imaging lab. This study was supported by NIH R01CA239288, Department of Defense W81XWH201055315, UCSF Loglio and NICO initiatives and the Hana Jabsheh Foundation. The authors acknowledge support from the NIH Center grant P41EB013598.References
- Shay, J. W. & Wright, W. E. Telomeres and telomerase: three decades of progress. Nature Reviews Genetics 20, 299-309, doi:10.1038/s41576-019-0099-1 (2019).
- Bell, R. J. et al. Understanding TERT Promoter Mutations: A Common Path to Immortality. Molecular cancer research : MCR 14, 315-323, doi:10.1158/1541-7786.mcr-16-0003 (2016).
- Sengupta, S. et al. Induced Telomere Damage to Treat Telomerase Expressing Therapy-Resistant Pediatric Brain Tumors. Molecular cancer therapeutics 17, 1504-1514, doi:10.1158/1535-7163.Mct-17-0792 (2018).
- Viswanath, P. et al. Non-invasive assessment of telomere maintenance mechanisms in brain tumors. Nature communications 12, 92, doi:10.1038/s41467-020-20312-y (2021).
- Schulte, M. L. et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat Med 24, 194-202, doi:10.1038/nm.4464 (2018).
- Mancini, A. et al. Disruption of the beta1L Isoform of GABP Reverses Glioblastoma Replicative Immortality in a TERT Promoter Mutation-Dependent Manner. Cancer cell 34, 513-528 e518 (2018).
- Giannini, C. et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-oncology 7, 164-176 (2005).
- Batsios, G. et al. Imaging 6-Phosphogluconolactonase Activity in Brain Tumors In Vivo Using Hyperpolarized δ-[1-(13)C]gluconolactone. Frontiers in oncology 11, 589570, doi:10.3389/fonc.2021.589570 (2021).
Figures