0283
Oxidation of ketone body in brain tumor patients1Kenneth R. Peak Brain and Pituitary Tumor Treatment Center, Department of Neurosurgery, Houston Methodist Research Institute, Houston, TX, United States, 2Weill Cornell Medical College, New York, NY, United States, 3Pathology and Genomic Medicine, Houston Methodist Research Institute, Houston, TX, United States
Synopsis
Normal brain switches to utilization of ketone bodies only during prolonged starvation. It is not known whether brain tumors can utilize ketone body under in vivo conditions. There are ongoing clinical trials on using ketogenic diet as adjuvant therapy for the treatment of brain tumors. Here, we show that brain tumors (meningioma and GBM) can fully oxidize ketone body and therefore ketogenic diet may not be effective in the treatment of these tumor patients. MCT1 is involved in the transport of BHB into tumor cells and therefore it is feasible to target BHB metabolism using MCT1 inhibitor.
Introduction
It is well known that the normal human brain uses ketone bodies as fuel during starvation. There are a few reports on using ketogenic diet in nutritional therapy to treat gliomas based on the Warburg’s observation that cancer cells often carry defective mitochondria and therefore cannot oxidize ketone bodies [1-6]. Also, there are ongoing clinical trials (ClinicalTrails.gov: NCT03160599, 03278249, 04461938) on using ketogenic diet as adjuvant therapy based on the above hypothesis. Although, our preliminary studies using patient-derived GBM cell lines clearly indicated that GBMs are fully capable of utilizing ketone body (β-hydroxybutyrate, BHB), in vivo metabolic fate of ketone bodies in brain tumor patients is unknown [7]. Here, we investigate oxidation of BHB in two different types of brain tumors (meningioma and glioma) through an innovative first-in-human in vivo metabolism protocol using 13C isotopomer technology.Methods
Brain tumor patients (meningioma and GBM) who were scheduled to go through surgical resection of their tumor mass were enrolled for this study after obtaining informed consent according to IRB protocol (Pro 00001457) approved by Houston Methodist Hospital IRB. Patients went through intravenous infusion of sterile/pyrogen free [U-13C]BHB with the bolus of 3.0 g for 10 minutes followed by 2.0 g/hour for 90 minutes during the surgery. Resected tumor tissues were immediately snap-frozen and are stored at -80° C. Tissues were extracted in 5% perchloric acid, lyophilized and was reconstituted 180 µL D2O containing 1.0 mM DSS (pH = 7.4). The solution was transferred to a 3-mm NMR tube, and 1H and 1H-decoupled 13C NMR spectra were collected on a Bruker 600 MHz spectrometer equipped with a 5-mm cryo-probe optimized for direct 13C detection. Using 13C isotopomer analysis of glutamate C4 and C3 13C multiplet signals, we determined the amount of 13C-labeled acetyl-CoA derived from the oxidation of BHB in the tumor cells. Blood samples were drawn at periodic intervals during the infusion to determine 13C plasma enrichment of BHB using 1H NMR spectroscopy. MCT1 histochemical staining was performed on fixed paraffin embedded tumor tissue slides. H&E and Ki-67 staining data from resected tumor specimens were also obtained from each patient.Results
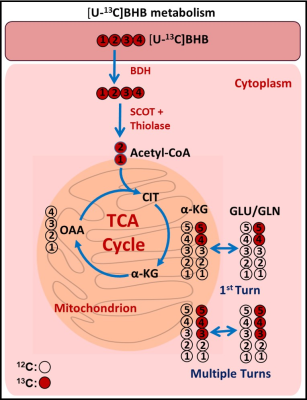
Figure 1 is a schema showing metabolism of [U-13C]BHB in the tumor cells. [U13C]BHB is converted to [U-13C]acetoacetate by the enzyme BHB dehydrogenase, and further leads to the production of [1,2-13C]acetyl-CoA. [U-13C]BHB-derived [1,2-13C]acetyl-CoA, in the first turn of the TCA cycle, forms 4 and 5 13C-labeled α-KG and glutamate (Figure 1). After multiple turns of the cycle, 3, 4, and 5 carbons of α-KG and glutamate are 13C-labeled. Figure 2 shows the portion of the 13C NMR spectrum with C4 multiplet signal of glutamate as inset. Doublet (D45) and the quartet (Q) are due [4,5-13C]glutamate, and [3,4,5-13C]glutamate respectively, which are the unequivocal readout of oxidation of [U-13C]BHB by tumor cells. We have so far enrolled 2 meningioma patients and a GBM patient for this study and are planning to enroll more patients. Our goal is to have data from at least six patients (3 meningiomas and 3 GBM) prior to the presentation at the meeting in May 2022. Both meningioma patients were oxidizing BHB in the TCA cycle leading up to 45.1% and 35.6% [1,2-13C]acetyl-CoA respectively (normalized with respect to their steady-state 13C-BHB plasma enrichment values). GBM patient tumor also showed evidence of BHB oxidation with lesser amount of [1,2-13C]acetyl-CoA production from BHB metabolism (10.3%). All the three patients showed MCT1 expression in their tumors. (Figure 3).Discussion
Current results from 3 brain tumor patients clearly prove that brain tumors of different types are fully capable of utilizing BHB as an alternative nutrient to meet their bioenergetic requirements. Though meningiomas show higher level of BHB oxidation via increased amount of [1,2-13C]acetyl-CoA compared to GBM, we need to have data from more patients to know whether meningiomas have higher propensity towards utilization of BHB than GBM.Conclusion
Current results from three tumor patients imply that ketogenic diet may not be effective in brain tumor patients. Also, these results suggest that targeting ketone body metabolism in brain tumor patients by MCT1 inhibitor (AZD3965) may be of therapeutic value.Acknowledgements
This study was supported by the Donna and Kenneth R. Peak Foundation, The Kenneth R. Peak Brain and Pituitary Tumor Treatment Center at Houston Methodist Hospital, The Houston Methodist Foundation, The Taub Foundation, The Pauline Sterne Wolff Foundation, The Veralan Foundation, The Marilee A. and Gary M. Schwarz Foundation, The John S. Dunn Foundation, Contributions in honor of Will McKone (DSB).References
1. H.T. Chang, L.K. Olson, K.A. Schwartz, Ketolytic and glycolytic enzymatic expression profile in malignant gliomas: implication for ketogenic diet therapy, Nutrient &Metabolism, 10 (2013) 47-53.
2. G.D. Maurer, D.P. Brucker, O. Bahr, P.N. Harter, E. Hattingen, S. Walenta, W.M-Klieser, J.P. Steinbach, J. Rieger, Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy, BMC Cancer, 11 (2011) 315-332.
3. T.N. Seyfried, J. Marsh, L.M. Shelton, L.C. Huysentruyt, P. Mukherjee, Is the restricted ketogenic diet a viable alternative to the standard of care for managing malignant brain cancer? Epilepsy Res. 100 (2012) 310-326.
4. A.C. Scheck, M.G. Abdelwahab, K.E. Fenton, P. Stafford, The ketogenic diet for the treatment of glioma: Insights from genetic profiling, Epilepsy Res. 100 (2012) 327-337.
5. L.C. Nebeling, F. Miraldi, S.B. Shurin, E. Lemer, Effects of a ketogenic diet on tumor metabolism and nutrional status in pediatric oncology patients: two case reports, J. Am. Coll. Nutr.14 (1995) 202-308.
6. S.G. Hasselbalch, G.M. Knudsen, J. Jakobsen, L.P. Hageman, S. Holm, O.B. Paulson, Brain metabolism during short-term starvation in humans, J. Cereb. Blood Flow Metab. 14 (1994) 125-31.
7. O.B. Ijare, A. Hoppe, C. Holan, M.A. Sharpe, D.S. Baskin, K. Pichumani, Human Glioblastoma Cell Lines Co-Oxidize [2,4-13C]betahydroxybutyrate and [U-13C]glucose: A 13C NMR Spectroscopic Study, Proc. Intl. Soc. Mag. Reson. Med. 26 (2018) 0450.
Figures
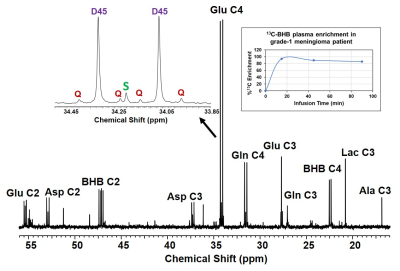
13C NMR spectrum of grade-1 meningioma tumor extract from a patient infused with [U-13C]BHB. Mitochondrial metabolism of [U-13C]BHB produces [4,5-13C]Glu (doublet, D45) and [3,4,5-13C]Glu (quartet, Q). Also shown is the plasma [U-13C]BHB levels during the infusion. Total of 6g of 13C-BHB was used in the patient that led to the ~80% 13C-BHB enrichment in the circulation at steady-state.
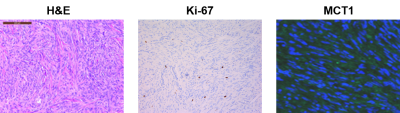
Histochemical staining images (H&E, Ki-67 and MCT1) of the grade-1 meningioma patient tumor. 13C NMR data of this patient is shown in Figure 2.