0281
Intracranial super-resolution 4D Flow MRI – using deep-learning to map flow and relative pressure in the brain1Massachusetts Institute of Technology, Cambridge, MA, United States, 2Karolinska Institutet, Stockholm, Sweden, 3University of Auckland, Auckland, New Zealand, 4University of Michigan, Ann Arbor, MI, United States, 5Northwestern University, Chicago, IL, United States, 6University of Greifswald, Greifswald, Germany, 7King's College London, London, United Kingdom
Synopsis
Changes in regional hemodynamics are indicative of cerebrovascular disease. However, image-based monitoring is complicated by the unique flow and anatomies found in the brain, with accurate estimates requiring beyond state-of-the-art image resolutions. To address this, we combine a deep residual network, 4D Flow MRI, and physics-informed image processing to provide super-resolution flow images and coupled accurate quantification of intracranial relative pressure. The method is trained and validated on patient-specific in-silico data, highlighting how low resolution-biases are mitigated by super-resolution conversion. Data were also effectively generated at <0.5 mm in a representative in-vivo cohort, highlighting the potential of our presented approach.
Introduction
Although 4D Flow MRI allows for full-field capture of blood flow throughout the brain1, its accuracy is highly dependent on spatial resolution2,3. The importance of resolution is particularly evident when deriving relative pressures from acquired images, and in previous work, we have shown that sub-mm resolution is necessary to achieve accurate estimations through the narrow and tortuous arterial cerebrovasculature3. Such fine resolutions are challenging to achieve in a reasonable time frame and at sufficient signal quality in a clinical setting. Since regional changes in flow and pressure are observed in a number of cerebrovascular diseases4,5, improved measures for high-resolution intracranial flow imaging are urgently required. The aim of this study was to evaluate whether a dedicated cerebrovascular super-resolution network could improve estimates of hemodynamic metrics through the brain, with specific attention to the derivation of image-based regional relative pressures.Methods
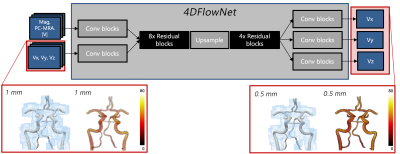
To achieve super-resolution flow imaging, the deep residual network 4DFlowNet6 was used. This architecture is based on a central upsampling layer surrounded by a series of stacked residual blocks, converting low resolution input image patches into de-noised, upsampled super-resolution equivalents (Fig. 1). With modifications introduced to adapt the original aortic network for cerebrovascular usage (12-cube input patch size; linear activation function output layer; loss function minimizing mean squared velocity errors across Cartesian components), the network was trained using an Adam optimizer with a learning rate of 10-4 and training completion at 60 epochs. Complete implementation was done in Tensorflow 2.07 with a Keras backend.To train the super-resolution network, synthetic 4D Flow MRI were generated from a calibrated set of patient-specific computational fluid dynamics (CFD) simulations of the arterial cerebrovasculature8. Four models scenarios were generated for patients with: Subject 1: no vascular disease; Subject 2: severe stenosis at the right proximal internal carotid artery (ICA); Subject 3a: bilateral ICA stenoses; Subject 3b: same as 3a after surgical re-opening of the right ICA. In each model, a region-of-interest was centered around the Circle of Willis (CoW), with synthetic 4D Flow MRI generated at varying spatial resolutions by sampling the CFD output onto a uniform voxelized image grid (dx = 0.5 to 1.5 mm). Data was further enhanced by adding realistic velocity-to-noise ratios and by extracting relevant clinical magnitude data from reference in-vivo scans. In total 42’900 patches (Subject 1 and 2) were assigned for training, 2’730 patches (Subject 3a) for validation and Subject 3b for testing. Training was performed on a Titan X GPU with 12GB memory, resulting in a total training time of ~30 hours.
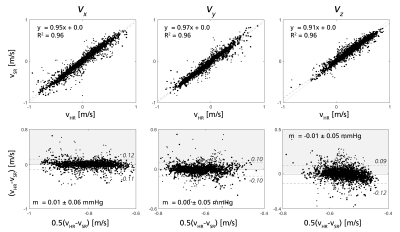
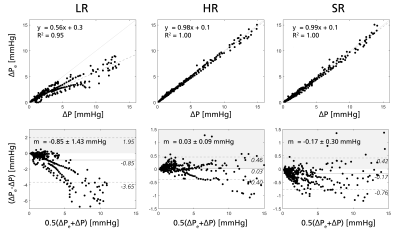
For validation, super-resolved velocities were compared against CFD-based high resolution references in linear regression and Bland-Altman form. Further, the virtual work-energy relative pressure (vWERP) method – shown in previous work to enable assessment of cerebrovascular relative pressures9 – was utilized across the CoW, extracting relative pressures in low, high, and super-resolution data, respectively.
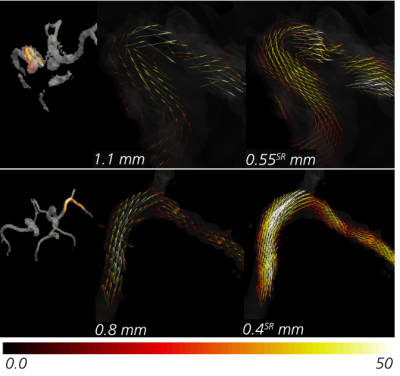
Lastly, to exemplify performance in a clinical setting, the super-resolution utility was applied on an in-vivo cohort of 8 healthy volunteers, with MRI acquired at 3T (Siemens Magnotom, Skyra) including a TOF MRA (TR = 21 ms; TE = 3.6 ms; flip angle = 18°) and a 4D Flow MRI (prospective k-t GRAPPA dual venc (130/45 cm/s)1, dt = 95-104 ms) sequence. Note that 4D Flow MRI was performed at both dx = 1.1 mm and dx = 0.8 mm in all subjects.
Results
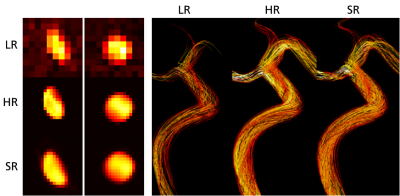
Figure 2 shows low, high, and super-resolved velocity fields through one of the in-silico models, highlighting significant noise-reduction and visually apparent upsampling. Figure 3-4 further shows linear regression and Bland-Altman representations for different velocity directions, and for derived relative pressures. Excellent correlations are observed between super- and high-resolution velocities (k>0.91; R2>0.96), with relative pressures extracted at similar accuracy (k>0.99; R2 = 1.00). Importantly, low-resolution bias in relative pressure estimates is effectively mitigated by super-resolution conversion. Lastly, Figure 5 shows exemplifying in-vivo super-resolution fields, estimated at different base and super-resolutions.Discussion
In this study, we demonstrated how super-resolution 4D Flow MRI effectively enables intracranial hemodynamic tracking using dedicated training data. We also showed how super-resolved 4D Flow MRI in combination with the physics-informed vWERP algorithm successfully recovers relative pressures through the Circle of Willis, resolving estimation bias otherwise observed in the low-resolution input data. A few studies exist attempting super-resolution flow recovery6,10, but only a handful have attempted coupled recovery of regional pressures11-12. Here, the combination of 4DFlowNet and vWERP shows particular promise in the intracranial space, adding to recent efforts exploring deep learning-enhanced imaging in the brain10,13. Whilst validation was performed on dedicated in-silico data, further work is needed to validate relative pressures in an in-vivo setting. Loss function optimization or architectural variations could be envisioned to further improve performance; however, it was not part of our current study.Conclusion
Through 4DFlowNet, intracranial super-resolution 4D Flow MRI is effectively enabled with cerebrovascular flow velocities and regional relative pressures accurately resolved at sub-mm scale. Specifically, biases associated with low-resolution data are effectively mitigated by super-resolution conversion and coupled image analysis (vWERP), indicating specific promise for future clinical usage. Current work is ongoing to validate behavior in a clinical setting, and to extend utilities to relevant patient cohorts.Acknowledgements
D.M. holds a Knut and Alice Wallenberg Foundation scholarship for postdoctoral studies at Massachusetts Institute of Technology. E. F. holds a New Zealand Heart Foundation Scholarship, Grant No. 1786. J.S. is supported by a University of Michigan Rackham Predoctoral Fellowship. M.A. was supported by a Ruth L. Kirschstein National Research Service Award (NIH F30 HL140910) and the Northwestern – Medical Science Training Program (NIH T32 GM815229). E.R.E. was funded in part by NIH R01 49039. A.A.Y. acknowledges core funding from the Wellcome/EPSRC Centre for Medical Engineering (WT203148/Z/16/Z) and the London Medical Imaging and AI Centre for Value-Based Healthcare. D.N. would like to acknowledge funding from the Engineering and Physical Science Research Council (EP/N011554 and EP/R003866/1)References
1. Schnell S, Ansari A, Wu C, et al. Accelarated dual-venc 4D flow MRI for neurovascular applications. JMRI. 2017:46:102-114
2. Aristova M, Vali A, Ansari SA, et al. Standardized evaluation of cerebral arteriovenous malformations using flow distribution network graphs and dual‐venc 4D flow MRI. JMRI. 2019:50:1718-1730
3. Marlevi D, Schollenberger J, Aristova M, et a. Noninvasive quantification of cerebrovascular pressure changes using 4D Flow MRI. MRM. 2021:86:3096-3110
4. Leng X, Wong KS, Liebeskind DS. Evaluating intracranial atherosclerosis rather than intracranial stenosis. Stroke. 2014:45:645-651
5. Penn DL, Komotar RJ, Connolly ES. Hemodynamic mechanisms underlying cerebral aneurysm pathogenesis. J Clinical Neuroscience. 2011:18:1435-1438
6. Ferdian E, Suinesiaputra A, Dubowitz DJ et al. 4DFlowNet: Super-resolution 4D Flow MRI using deep learning and computational fluid dynamics. Frontiers in Physics. 2020:8:138
7. Abadi M, Barham P, Chen J, et al. Tensorflow: A system for large-scale machine learning, in 12th {USENIX} symposium on operating systems design and implementation. 2016:265-283.
8. Schollenberger J, Osborne NH, Hernandez-Garcia L, et al. A combined computational fluid dynamics and MRI Arterial Spin Labeling modeling strategy to quantify patient-specific cerebral hemodynamics in cerebrovascular occlusive disease. Front Bioeng Biotech. 2021:689
9. Marlevi D, Ruijsink B, Balmus M, et al. Estimation of Cardiovascular Relative Pressure Using Virtual Work-Energy. Sci Rep. 2019:9:1375
10. Rutkowski DR, Roldán-Alzate A, Johnson KM. Enhancement of cerebrovascular 4D flow MRI velocity fields using machine learning and computational fluid dynamics simulation data. Sci Rep. 2021:11:1-11
11. Kissas G, Yang Y, Hwuang E, et al. Machine learning in cardiovascular flows modeling: Predicting arterial blood pressure from non-invasive 4D flow MRI data using physics-informed neural networks. Comp Meth Appl Mech Eng. 2020:358:112623
12. Shit S, Das D, Ezhov I, et al. Velocity-To-Pressure (V2P)-Net: Inferring Relative Pressures from Time-Varying 3D Fluid Flow Velocities. Int Conf Inf Proc Med Im. 2021:545-558.
13. Fathi MF, Perez-Raya I, Baghaie A, et al. Super-resolution and denoising of 4D-Flow MRI using physics-Informed deep neural nets. Comp Meth Progr Biomed. 2020:197:105729
Figures