0280
Time-delay processing of cerebrovascular reactivity reveals pre-surgical indicators of revascularization response in adults with moyamoya1Department of Neurology, Vanderbilt University Medical Center, Nashville, TN, United States, 2Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 3Department of Neurosurgery, Vanderbilt University Medical Center, Nashville, TN, United States, 4Department of Pediatrics, Division of Pediatric Neurology, Vanderbilt University Medical Center, Nashville, TN, United States, 5Department of Psychiatry and Behavioral Sciences, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
The goal of this work is to apply time-regression analysis to hypercapnic reactivity data in an interventional trial to identify pre-surgical indicators of treatment response in moyamoya vasculopathy patients. We hypothesized that pre-surgical posterior flow-territory reactivity, including greater cerebrovascular reactivity and reduced vascular delay time, portend collateralization success. In 41 participants evaluated pre and 1-year post-operatively, pre-surgical posterior flow-territory maximum reactivity was higher (p=0.02) in patients with good vs. poor angiographic outcomes. This highlights that topographical features of preserved cerebral auto-regulation may contribute to neoangiogenic potential, and importantly, that reactivity mapping may provide a diagnostic tool for informing surgical candicacy.
Introduction
The overarching goal of this work is to apply a novel time-regression analysis to hypercapnic cerebrovascular reactivity data in a longitudinal interventional trial to identify pre-surgical indicators of treatment response in patients with intracranial moyamoya vasculopathy. Moyamoya disease (MMD) is a cerebrovascular condition characterized by frequently progressive non-atherosclerotic arterial stenosis of the anterior intracranial segments of the internal carotid arteries (ICA), especially the middle cerebral arteries (MCA) (1,2). Appropriate MMD management typically includes surgical revascularization with direct revascularization (3), indirect revascularization (2), or a combination thereof. However, not all persons with MMD who undergo surgical revascularization have similar or successful outcomes. To improve informed treatment selection, we aimed to identify whether personalized functional tissue signatures may portend surgical response. The hypothesis investigated is that pre-surgical hemodynamic MRI profiles, specifically higher posterior flow-territory cerebrovascular reactivity (CVR) and reduced CVR response times following a vasoactive stimulus, portend revascularization outcomes at one-year follow-up.Methods
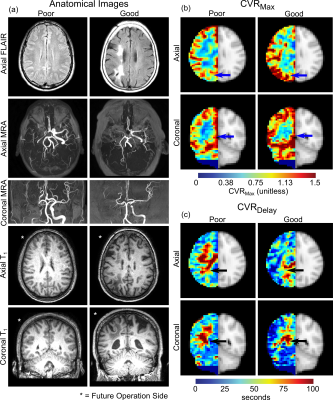
Participants. Adult North American participants with moyamoya who were scheduled for clinically-indicated surgical revascularization provided informed, written consent and underwent hemodynamic MRI pre-surgically, as well as digital subtraction angiography (DSA) before and one year after surgical revascularization. Non-imaging stroke risk factors including, but not limited to, age, sex, race, vasculopathy extent, and type 2 diabetes status were recorded.MRI. MRI studies were performed at 3.0T (Philips Healthcare). Anatomical images included standard anatomical diffusion-weighted, T2-weighted, T2-weighted axial fluid-attenuated inversion recovery (FLAIR), and T1-weighted scans. Pre-surgical CVR-weighted images were obtained during BOLD imaging (TR/TE=2000/30 ms; spatial resolution=3.0x3.0x3.5 mm3) with a vasodilatory hypercapnic-stimulus delivered at 12 L/min, designed to elicit a mean 6 mmHg change in end-tidal CO2.
Image Analysis. Post-surgical arterial collateral grading was performed by a board-certified neuroradiologist using DSA, and was used to classify participant hemispheres according to good or poor responses (4,5). Poor response was defined as less than 1/3 MCA territory collateralization, and good response as greater than 1/3 MCA territory collateralization. Two CVR parameters were calculated from BOLD reactivity data (6). Time-to-maximum correlation was defined as CVRDelay (seconds), and the value of the maximum correlation as CVRMax (whole-brain normalized z-statistic between shifted regressor and voxel time course). Values were calculated on a voxel-wise basis to generate CVRDelay (time delay) and CVRMax (maximal statistical response) maps. Mean parameter values were taken from the vertebrobasilar (VBA) flow territory to evaluate posterior circulation hemodynamics. Statistical analysis. A Wilcoxon rank-sum test was applied to evaluate differences in VBA flow-territory CVRDelay and CVRMax between those with good vs. poor surgical responses. Significance was defined as two-sided p<0.05 (Bonferroni corrected p<0.025).
Results
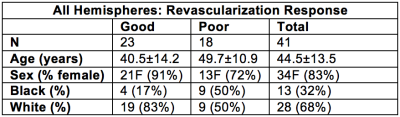
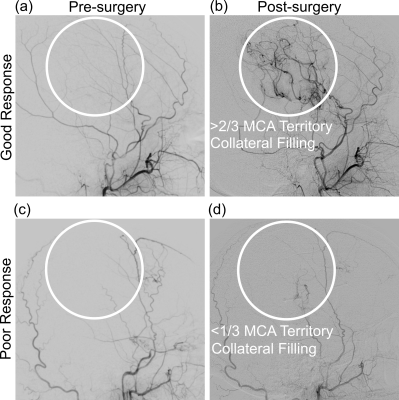
Demographics. MR imaging and longitudinal angiography was performed on 31 participants (sex= 26F / 5M; age= 45.2±12.5 years), from which 41 total revascularized hemispheres were considered (Table 1).Revascularization response. For post-surgical follow-up, DSA was acquired at 12.6±7.5 months post-surgery to evaluate collateral filling of the MCA territory. Figure 1 illustrates the categorical scoring criteria. Of the 41 hemispheres included, 23/41 had poor responses and 18/41 had good responses at follow-up.
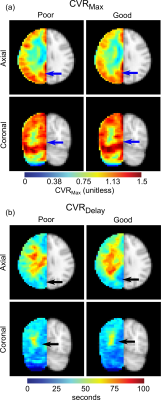
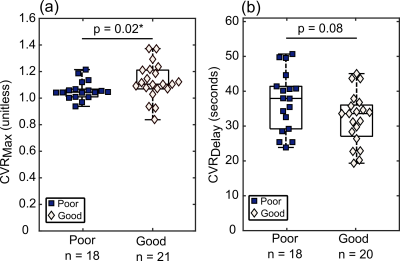
Pre-surgical indicators of revascularization success. Maximum CVR in the VBA flow-territory (CVRMax) was reduced in those meeting angiographic criteria for poor (1.06 ± 0.07) vs. good (1.13 ± 0.13) responses (p=0.02). A trend was observed for mean CVRDelay being reduced in participants meeting angiographic criteria for good (37.1±8.5 seconds) vs. poor (32.2±7.4 seconds) responses, but this did not meet criteria for significance (p=0.08). These effects are demonstrated on group-averaged reactivity maps in Figure 2 and quantitative values are in Figure 3. Figure 4 summarizes examples of a 28-year-old white female meeting criteria for good response to surgery and a 28-year-old white male meeting criteria for a poor response to surgery.
Discussion
This study utilized novel time-regression analyses of hypercapnic reactivity data, acquired during clinically-indicated diagnostic procedures, to evaluate the relevance of hemodynamic tissue signatures for predicting surgical outcomes in patients with intracranial vasculopathy. We provide evidence for hemodynamic parameters, specifically higher posterior territory reactivity, having potential for portending favorable revascularization responses.The VBA flow-territory, i.e. the posterior cerebral circulation, is typically less affected until advanced stages of moyamoya (7). Here, greater posterior flow-territory CVRMax, and reductions in CVRDelay, in participants who responded better to surgical intervention provide evidence that participants with these reactivity profiles may have higher collateralization potential. It is important to note that these VBA flow-territory changes indicate potential for MCA territory reperfusion, not necessarily that these posterior flow territories themselves are developing additional collateral networks in response to surgical revascularization. Prior literature indicates that as vasculopathy progresses, tissue can be supplied by complex networks of arterial collaterals, which are increasingly supplied by the posterior circulation, the communicating arteries, and the ophthalmic artery (8,9).
Conclusion
We provide evidence for pre-surgical VBA flow-territory cerebrovascular reactivity being greater in moyamoya participants with greater arterial collateralization in the MCA territory at one-year follow up after indirect surgical revascularization. This highlights that topographical features of preserved cerebral reserve capacity in moyamoya may contribute to neoangiogenic potential, and importantly, that non-invasive cerebrovascular reactivity mapping may provide a diagnostic tool for informing surgical candicacy in these patients, in whom no definitive diagnostic tests for predicting outcomes are currently available.Acknowledgements
No acknowledgement found.References
1. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med 2009;360(12):1226-1237.
2. Scott RM. Moyamoya syndrome: a surgically treatable cause of stroke in the pediatric patient. Clin Neurosurg 2000;47:378-384.
3. Zaharchuk G, Do HM, Marks MP, Rosenberg J, Moseley ME, Steinberg GK. Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke 2011;42(9):2485-2491.
4. Watchmaker JM, Frederick BD, Fusco MR, et al. Clinical Use of Cerebrovascular Compliance Imaging to Evaluate Revascularization in Patients With Moyamoya. Neurosurgery 2018.
5. Mugikura S, Takahashi S, Higano S, Shirane R, Sakurai Y, Yamada S. Predominant involvement of ipsilateral anterior and posterior circulations in moyamoya disease. Stroke 2002;33(6):1497-1500.
6. Donahue MJ, Dethrage LM, Faraco CC, et al. Routine clinical evaluation of cerebrovascular reserve capacity using carbogen in patients with intracranial stenosis. Stroke 2014;45(8):2335-2341.
7. Hishikawa T, Tokunaga K, Sugiu K, Date I. Clinical and radiographic features of moyamoya disease in patients with both cerebral ischaemia and haemorrhage. Br J Neurosurg 2013;27(2):198-201.
8. Vernieri F, Pasqualetti P, Matteis M, et al. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke 2001;32(7):1552-1558.
9. Strother MK, Anderson MD, Singer RJ, et al. Cerebrovascular collaterals correlate with disease severity in adult North American patients with Moyamoya disease. AJNR Am J Neuroradiol 2014;35(7):1318-1324.
Figures