0276
Interleaved 23Na/1H MRI of the human heart at 7 Tesla1Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 3Faculty of Electrical Engineering and Information Technology, University of Applied Sciences, Aachen, Germany, 4Rapid Biomedical GmbH, Rimpar, Germany, 5Fraunhofer MEVIS, Bremen, Germany, 6Department of Nephrology and Hypertension, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany
Synopsis
A dual-nuclear interleaved 23Na/1H MRI sequence for cardiac MRI was implemented and evaluated in phantom and in vivo measurements using a 23Na body coil in combination with two 4 Tx/8 Rx 1H arrays. The 1H arrays were operated in 1Tx mode with fixed transmit magnitude/phase setting. Compared to single-nuclear sequences, the interleaved sequence led to almost identical SNR und image intensities in phantom measurements. Furthermore, the feasibility of interleaved 23Na/1H in vivo MRI measurements at 7 T was demonstrated. The interleaved approach enables reduced acquisition times and further eliminates the need for image co-registration.
Introduction
Sodium ions (23Na+) are involved in various biological processes and in many cases the tissue 23Na concentration is closely linked to cell viability. For example, increased 23Na MR signal is visible in nonviable myocardial tissue after myocardial infarction 1. Therefore, 23Na MRI can give additional diagnostic information about dysfunctional myocardium and could be used to monitor therapeutic measures 2.Due to the lower signal-to-noise ratio (SNR) of 23Na MRI compared to 1H MRI, additional high-resolution 1H images are necessary to segment cardiac compartments for partial volume correction 3 of the 23Na MRI data as well as for quantitative evaluations 2. Since 23Na and 1H scans are usually performed consecutively, the total acquisition time is elongated and the analysis is also prone to patient motion during or between the 23Na and 1H measurements. A 23Na body coil combined with an 1H transmit/receive array and an interleaved 23Na/1H sequence scheme could enable the acquisition of cardiac 23Na and 1H images within one single scan. This approach could reduce the total acquisition time and further ensure that respiratory or cardiac sorted 23Na and 1H images represent the quasi-same physiological states.
Methods
HardwareAll measurements were performed on a whole-body 7 Tesla system (MAGNETOM Terra, Siemens Healthineers, Erlangen, Germany), using a dual-tuned 23Na/1H coil (RAPID Biomedical, Rimpar, Germany). The coil consists of a 23Na volume coil and two 4 Tx/ 8 Rx 1H body arrays (see Figure 1), which were driven in 1Tx mode with a vendor provided heart shim 4. The 23Na volume coil is constructed as a four-rung birdcage with asymmetric end-rings adjusted to the magnet bore for providing maximum space and comfort for the patient. The setup allows interleaved acquisition of 23Na/1H MR data within a single scan.
Measurements
A dual-nuclear interleaved 5 pulse sequence with a density-adapted 3D radial readout scheme (DA-3D-RAD) 6 was employed to acquire 23Na and 1H images. The sequence scheme is shown in Figure 2 and the sequence parameters are given in Table 1. First, the 23Na signal is acquired. Afterwards the remaining idle time of each 23Na TR is used for the acquisition of a 1H image. Within each 23Na TR four 1H projections were acquired. Both 23Na and 1H data were zerofilled to a voxel size of (1 mm)3 and radial k-space data were acquired in a golden angle scheme 7 to allow retrospective respiratory 8 and cardiac gating 2 in the future. So far, all projections were included in the gridding reconstruction 9. For correction of the receive profile of the 1H array, a method using a support region as described in 10 was applied to the in vivo 1H images.
To investigate possible influences of the interleaved acquisition on the 23Na/1H images, phantom measurements were performed with a single-nuclear as well as with the described dual-nuclear interleaved sequence and compared to each other. The single-nuclear sequences were implemented by turning off excitation and readout gradients of one nucleus (23Na or 1H) in the interleaved sequence. Furthermore, the interleaved sequence was tested on a healthy volunteer (male, 24) in compliance with the institutional policies and with approval of the local ethics committee.
Results
Figure 3 shows a comparison of the 23Na and 1H phantom images acquired with the dual-nuclear interleaved and the single-nuclear sequence. The mean difference between interleaved and single image was -0.11 % $$$\pm$$$ 0.31 % for 23Na and -0.06 % $$$\pm$$$ 0.06 % for 1H. Furthermore, the SNR obtained over the whole phantom was almost identical for interleaved and single acquisition (23Na: 22.9 versus 23.6; 1H: 5.3 versus 5.3).An in vivo interleaved 23Na/1H MRI measurement (TA = 12:30 min) of a healthy volunteer is shown in Figure 4. The 23Na image shows a reduced 23Na signal within the myocardium compared to blood (see arrows in a). In general, the myocardium can be identified in both images (a: interleaved 23Na MRI, b: interleaved 1H MRI). Due to the interleaved acquisition, both images are on top of each other (c) without the need for co-registration.
Discussion and Conclusion
In this work, we demonstrated that the presented dual-nuclear 23Na/1H interleaved sequence led to almost identical images as the corresponding 23Na and 1H single-nuclear sequences. Therefore, an interleaved acquisition might also not influence the quantification of 23Na concentrations. Additionally, the interleaved 23Na/1H acquisition ensures that both images – also in case of respiratory or cardiac sorting – represent the quasi-same physiological states and patient position. This might not be the case for consecutive 23Na and 1H acquisition due to patient movement in one of or in between the measurements. Furthermore, the interleaved scheme could enable the acquisition of 1H navigator images 11 to improve respiratory sorting of the 23Na data. Since interleaved measurements are not yet possible in parallel transmission (pTx) mode of the scanner, a vendor provided heart shim was used for 1H imaging. However, this leads to B1+ inhomogeneity in the 1H images, hence the combination of 23Na and 1H imaging in pTx mode could be beneficial. Overall, the interleaved acquisition of in vivo cardiac 23Na/1H images at 7 T is feasible within clinically acceptable scan times.Acknowledgements
This project was funded by the Deutsche Forschungsgemeinschaft (DFG) under NA 736/6-1.References
1. Sandstede JJ, Hillenbrand H, Beer M, Pabst T, Butter F, Machann W, et al. Time course of 23Na signal intensity after myocardial infarction in humans. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2004;52(3):545-51.2. Lott J, Platt T, Niesporek SC, Paech D, GR Behl N, Niendorf T, et al. Corrections of myocardial tissue sodium concentration measurements in human cardiac 23Na MRI at 7 Tesla. Magnetic resonance in medicine. 2019;82(1):159-73.
3. Niesporek SC, Hoffmann SH, Berger MC, Benkhedah N, Kujawa A, Bachert P, et al. Partial volume correction for in vivo 23Na-MRI data of the human brain. Neuroimage. 2015;112:353-63.
4. Reiter T, Lohr D, Hock M, Ankenbrand MJ, Stefanescu MR, Kosmala A, et al. On the way to routine cardiac MRI at 7 Tesla-a pilot study on consecutive 84 examinations. Plos one. 2021;16(7):e0252797.
5. de Bruin PW, Koken P, Versluis MJ, Aussenhofer SA, Meulenbelt I, Börnert P, et al. Time‐efficient interleaved human 23Na and 1H data acquisition at 7 T. NMR in Biomedicine. 2015;28(10):1228-35.
6. Nagel AM, Laun FB, Weber MA, Matthies C, Semmler W, Schad LR. Sodium MRI using a density-adapted 3D radial acquisition technique. Magn Reson Med. 2009;62(6):1565-73.
7. Chan RW, Ramsay EA, Cunningham CH, Plewes DB. Temporal stability of adaptive 3D radial MRI using multidimensional golden means. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2009;61(2):354-63.
8. Platt T, Umathum R, Fiedler TM, Nagel AM, Bitz AK, Maier F, et al. In vivo self‐gated 23Na MRI at 7 T using an oval‐shaped body resonator. Magnetic resonance in medicine. 2018;80(3):1005-19.
9. Jackson JI, Meyer CH, Nishimura DG, Macovski A. Selection of a convolution function for Fourier inversion using gridding (computerised tomography application). IEEE transactions on medical imaging. 1991;10(3):473-8.
10. Lachner S, Ruck L, Niesporek SC, Utzschneider M, Lott J, Hensel B, et al. Comparison of optimized intensity correction methods for 23Na MRI of the human brain using a 32-channel phased array coil at 7 Tesla. Zeitschrift für Medizinische Physik. 2020;30(2):104-15.
11. Firmin D, Keegan J. Navigator echoes in cardiac magnetic resonance. Journal of Cardiovascular Magnetic Resonance. 2001;3(3):183-93.
Figures
Table 1: Parameters of the interleaved 23Na/1H sequence. For phantom and in vivo measurements different numbers of projections were acquired and pulses with different nominal flip angles (due to specific absorption rate constraints) were used. For the 1H data there are two different repetition times TR1H,a and TR1H,b (see Figure 2). Due to the interleaved sequence scheme, both 23Na and 1H data were acquired within the acquisition time of 12:30 min.
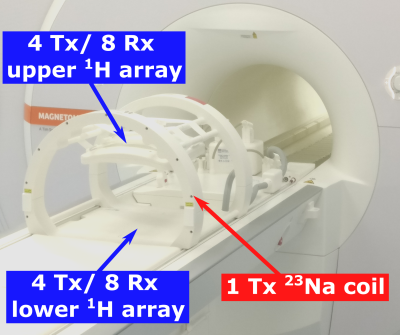
Figure 1: Measurement setup: The 1H 8 Tx/ 16 Rx body array consists out of an upper 4 Tx/ 8 Rx and a lower 4 Tx / 8 Rx array, which are placed on top of the chest and below the back of the patient, respectively. The 1H body array was used in 1Tx mode with a fixed cardiac phase shim. The dimensions of the 23Na volume coil are precisely matched to the diameter of the bore of the MR scanner.

Figure 2: Interleaved 23Na/1H sequence scheme: After a rectangular pulse (Tx) with duration Tpulse,23Na, one 23Na projection is acquired (Rx) with readout duration TRO,23Na. During the idle time of the 23Na repetition time TR23Na, four 1H projections are acquired. After the acquisition of each 23Na or 1H projection a rewinder and spoiler gradient in z-direction are played out. The latter is particularly important in the case of a golden angle acquisition.

Figure 3: Comparison of the dual-nuclear interleaved (top) and a single-nuclear sequence (middle). All images were normalized to the maximum. The comparison shows similar SNRs for both sequences as well as only slight deviations in the images (bottom).
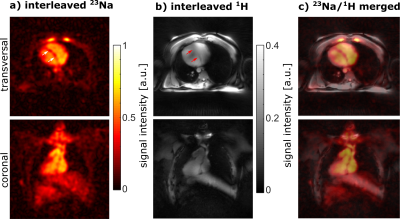
Figure 4: Interleaved 23Na/1H in vivo measurements (TA = 12:30 min, FoV = (420 mm)3) of a healthy volunteer. The myocardium is visible in the transversal 23Na (a) and 1H image (b). The merged images (c) illustrate that 23Na and 1H images are on top of each other without the need for image co-registration. However, the 1H image shows B1+ inhomogeneities, as the 1H array was used in 1Tx mode with a fixed heart shim, which was therefore not individually optimized for the volunteer.