0274
3D Joint Reconstruction of Non-Contrast and Contrast-Enhanced CMR Multitasking1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 3Department of Bioengineering, University of California in Los Angeles, Los Angeles, CA, United States, 4Siemens Medical Solutions USA, Los Angeles, CA, United States
Synopsis
Cardiovascular MR (CMR) is used to acquire function, morphology, and composition of the heart which aids in clinical diagnosis and management. While measures such as ejection fraction, ventricular dimensions, and presence/pattern of late gadolinium enhancement (LGE) have stood the test of time as diagnostic pillars, newer quantitative tissue property measurements such as T1, T2, and extracellular volume (ECV) can characterize diffuse fibrosis, inflammation, or infiltration. The purpose of this study was to integrate multiple quantitative measurements into a single 20-min free-breathing, non-ECG, motion-resolved whole ventricular 3D acquisition using CMR Multitasking, including cardiac function, pre-contrast T1/T2, ECV, and LGE images.
Introduction
Quantitative CMR (e.g., T1/T2/ECV/LGE) requires a series of consecutive pulse sequences which require breath-holding and ECG gating. This results in extended scan duration, risk of motion artifacts, and potential misalignment between separate sequences. Quantitative CMR techniques such as MR fingerprinting1 and multiparametric mapping techniques2-4 can jointly acquire pre-contrast T1 and T2 maps, but typically still require ECG and/or breath-holding. In this work, joint reconstruction of 3D CMR Multitasking5,6 data transforms the CMR exam from a series of multiple breath-hold, ECG-triggered to a single 20-min free-breathing scan without the need for cardiac gating. This single acquisition provides co-registered cardiac function, T1, T2, ECV, and LGE images, decreasing exam time by 33-50%, and simplifying the workflow.Methods
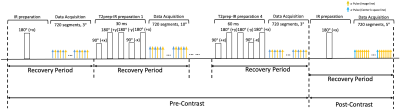
Sequence Design: A prototype 3D stack-of-stars FLASH pulse sequence was modified to collect image data incremented by a tiny golden angle (23.67°) interleaved with subspace training data acquired at the central 0° spoke every 8th readout. In the first half of the scan (pre-contrast), interleaved IR and T2prep-IR preparation pulses are employed to generate T1 and T2 contrasts (Fig. 1). Every other recovery period, FLASH excitation alternates between two flip angles (e.g., 3° and 10°) to allow for B1+ field corrected T1 mapping7. In the second half of the scan, immediately prior to Gd injection, the preparation scheme switches to IR-only to allow for ECV mapping and post-contrast phase-sensitive inversion recovery (PSIR) imaging.Image Model: In order to provide co-registered pre- and post-contrast maps, we jointly reconstructed all images from one 20-min acquisition spanning pre- and post-contrast (10 mins “pre” + 10 mins “post”) using the Multitasking framework5,6. The 10-min post-contrast acquisition was time-resolved to track the settling of Gd into a steady-state, with the last time point used for post-contrast measures such as ECV and LGE.
Pre-contrast images were represented as a 6-way tensor $$$\mathcal{A}_{pre}$$$ with a combined spatial dimension and 5 time dimensions (T1 recovery, T2prep duration, flip angle, cardiac motion, and respiratory motion). Post-contrast images were a 5-way tensor $$$\mathcal{A}_{post}$$$ with a combined spatial dimension and 4 time dimensions (T1 recovery, cardiac motion, respiratory motion, and DCE). Spatiotemporal correlation induces both tensors to be low-rank and to belong to a shared spatial subspace, such that $$$\mathcal{A}_{pre,(1)}= \Phi_{pre} \times_1 \mathbf{U}$$$ and $$$\mathcal{A}_{post,(1)}=\Phi_{post} \times_1 \mathbf{U}$$$. The matrix $$$\mathbf{U} $$$ spans this shared spatial subspace, and the $$$\Phi$$$’s are low-rank tensors constructed as weighted outer products of bases for each time dimension.
Image Reconstruction: Modified k-means clustering5 identified 6 respiratory bins and 20 cardiac phases. Bin-to-bin translational image registration compensated for respiratory motion and aided co-registration of all images. Data during the first-pass of Gd were excluded from binning and image reconstruction, and subsequent dynamic contrast enhancement was assigned to 1min-long bins. Following binning and motion compensation, $$$\Phi_{pre}$$$ and $$$\Phi_{post}$$$ were estimated from the subspace training data using Bloch-constrained low-rank tensor completion5. was then recovered by:
$$\hat{\mathbf{U}} = \underset{\mathbf{U}}{\operatorname{argmin}} ||\mathbf{d}_{pre}-\Omega_{pre}(\Phi_{pre} \times_1 \mathbf{FSU})||^2 + ||\mathbf{d}_{post}-\Omega_{post}(\Phi_{post} \times_1 \mathbf{FSU})||^2 + R(\mathbf{U})$$
where the $$$\mathbf{d}$$$’s are measured data, $$$\Omega$$$’s are undersampling operators, $$$\mathbf{F}$$$ is the nonuniform Fourier transform operator, $$$\mathbf{S}$$$ is the coil sensitivity operator, and $$$R(\cdot)$$$ is a regularization functional promoting transform sparsity (in this case, wavelet sparsity of $$$\mathbf{U}$$$).
Data Collection: Five human volunteers (42$$$\pm$$$26 yrs) were imaged on a 3T scanner (MAGNETOM Vida, Siemens). Multitasking pulse sequence parameters were: TR/TE=3.6ms/1.7ms, recovery period=2.5s, spatial resolution=1.4x1.4x8.0mm3. Total scan time was 20:20min (9:29pre/10:51post) for all subjects. The gadolinium dose is 0.2mmol/kg. Reference pre-/post-contrast 2D T1 maps with MOLLI and pre-contrast 2D T2 maps with T2-prep FLASH (1.4x1.4x8.0mm3) were acquired at both systole and diastole during end-expiration breath-holds. Full short-axis stacks of ECG-gated, breath-held, 2D bSSFP cine scans (1.3x1.3x8.0mm3, pre-contrast) and PSIR scans (1.4x1.4x8.0mm3, post-contrast) were acquired.
Results
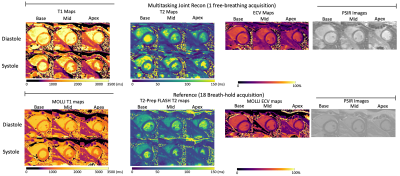
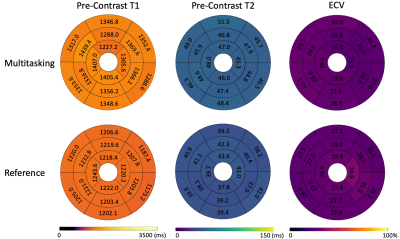
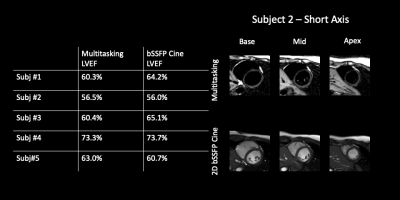
T1/T2/ECV mapping results and PSIR images from one healthy subject are shown in Fig. 2. Figs. 3 and 4 depict the summary statistics for segment-wise T1/T2/ECV values. Multitasking measured higher myocardial T1 values (1343$$$\pm$$$125.1ms) than MOLLI (1212$$$\pm$$$45.2ms), which is known to underestimate T18. Multitasking measured higher myocardial T2 values (47.1$$$\pm$$$10.2ms) than the T2-prep FLASH method (40.2$$$\pm$$$5.1ms), which is consistent with previous 3D Multitasking observations6 and is still within the normal range reported by some studies9-11. Multitasking ECV values (27.9$$$\pm$$$3.2%) were similar to MOLLI ECV (28.5$$$\pm$$$4.1%), and were all within the normal range12. LGE was not present in any of these healthy volunteers. Fig. 5 shows the black-blood Multitasking cines and bright-blood bSSFP cines; both produced comparable LVEF values (64.4%$$$\pm$$$6.3% for reference, 62.2%$$$\pm$$$6.4% for Multitasking).Discussion
A conventional examination including 9-to-12 slices for cardiac function and LGE, and 3 slices each of T1/T2/ECV would result in 27-33 breath-holds and a total exam time of ~30-40min. Multitasking reduced overall exam time to 20min, all free-breathing with no ECG reliance. Furthermore, Multitasking images and maps are all co-registered, potentially simplifying post-processing. Future work will focus on addressing partial volume effects by increasing the spatial resolution, optimizing slice orientation relative to the heart, or using the thicker systolic myocardium for measurements.Conclusion
Joint reconstruction of pre-contrast and post-contrast data is feasible with CMR Multitasking. This method is promising for the single-scan acquisition of quantitative CMR parameters in 20 minutes. Larger subject cohorts including cardiomyopathy patients are necessary for further investigation.Acknowledgements
The authors acknowledge with gratitude the support of Cedars-Sinai Medical Center Research Imaging Core staff. This work was supported by the National Institutes of Health grant R01 EB028146.References
[1] Ma, D., Gulani, V., Seiberlich, N., Liu, K., Sunshine, J. L., Duerk, J. L., & Griswold, M. A. (2013). Magnetic resonance fingerprinting. Nature, 495(7440), 187-192.
[2] Qi, H., Bustin, A., Cruz, G., Jaubert, O., Chen, H., Botnar, R. M., & Prieto, C. (2019). Free-running simultaneous myocardial T1/T2 mapping and cine imaging with 3D whole-heart coverage and isotropic spatial resolution. Magn Reson Imaging, 63, 159-169.
[3] Guo, R., Chen, Z., Herzka, D. A., Luo, J., & Ding, H. (2019). A three‐dimensional free‐breathing sequence for simultaneous myocardial T1 and T2 mapping. Magn Reson Med, 81(2), 1031-1043.
[4] Milotta, G., Bustin, A., Jaubert, O., Neji, R., Prieto, C., & Botnar, R. M. (2020). 3D whole‐heart isotropic‐resolution motion‐compensated joint T1/T2 mapping and water/fat imaging. Magn Reson Med, 84(6), 3009-3026.
[5] Christodoulou, A. G., Shaw, J. L., Nguyen, C., Yang, Q., Xie, Y., Wang, N., & Li, D. (2018). Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nature BME, 2(4), 215-226.
[6] Mao, X., Serry, F. M., Ma, S., Hu, Z., Kwan, A. C., Han, F., Lee, H.-L., Xie, Y., Li, D., & Christodoulou, A. G. (2021). 3D Whole-ventricle, Free-Breathing, Non-ECG, T1-T2-B1+ Mapping and Cine Imaging with Cardiac MR Multitasking, in Proceedings of International Society of Magnetic Resonance and Medicine (ISMRM), 30th Annual International Conference.
[7] Serry, F., Ma, S., Mao, X., Han, F., Xie, Y., Han, H., Li, D., & Christodoulou, A. (2021). Dual flip angle (2FA) IR-FLASH with spin history mapping for B1+-insensitive T1 mapping: Application to T1 cardiovascular magnetic resonance multitasking, Magn Reson Med, In Press.
[8] Kellman, P., & Hansen, M. S. (2014). T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson, 16(1), 1-20.
[9] Snel, G. J. H., Van Den Boomen, M., Hernandez, L. M., Nguyen, C. T., Sosnovik, D. E., Velthuis, B. K., ... & Prakken, N. H. J. (2020). Cardiovascular magnetic resonance native T2 and T2* quantitative values for cardiomyopathies and heart transplantations: a systematic review and meta-analysis. J Cardiovasc Magn Reson, 22, 1-34.
[10] An, D. A., Chen, B. H., Shi, R. Y., Bu, J., Ge, H., Hu, J., ... & Wu, L. M. (2018). Diagnostic performance of intravoxel incoherent motion diffusion‐weighted imaging in the assessment of the dynamic status of myocardial perfusion. J Magn Reson Imaging, 48(6), 1602-1609.
[11] Gang, L., Mu, Z., Zenglin, M., Jiayi, L., Zhanming, F., Dongting, L., & Zhaoying, W. (2018). Cardiac magnetic resonance quantitative tissue markers in the clinical application value for the diagnosis of acute myocarditis. J Med Imaging & Health Infor., 8(9), 1751-1756.
[12] Heidenreich, J. F., Weng, A. M., Donhauser, J., Greiser, A., Chow, K., Nordbeck, P., ... & Köstler, H. (2019). T1-and ECV-mapping in clinical routine at 3 T: differences between MOLLI, ShMOLLI and SASHA. BMC Med Imaging, 19(1), 59.
Figures