0273
Adiabatic T1ρ mapping of the human myocardium at 3T.1Department of Imaging Physics, TU Delft, Delft, Netherlands, 2Department of Electrical and Computer Engineering, University of Minnesota, Minneapolis, MN, United States, 3Medical Imaging and Radiation, Holland Proton Therapy Center, Delft, Netherlands
Synopsis
T1ρ-mapping constitutes a promising contrast-free alternative to LGE MRI for assessment of myocardial viability. However its applicability is hindered by susceptibility to B0 and B1+ field imperfections. In this work we propose a single breath-hold ECG-triggered adiabatically-prepared single-shot bSSFP T1ρ-mapping sequence. Bloch simulations are employed to identify optimal parameters for adiabatic pulses. The use of adiabatic preparations yields better resilience to system inhomogeneities and improved myocardium/blood contrast compared with conventional spin-lock modules, both in phantom and in-vivo. Thus, adiabatically-prepared sequences at 3T form a promising candidate for non-contrast evaluation of ischemic and non-ischemic cardiomyopathies.
Introduction
Late gadolinium enhancement is the clinical gold standard for the assessment of myocardial viability. However, the need for exogenous contrast agents limits its repeated use and the clinical applicability in patients with contraindications. T1ρ-mapping has emerged as a promising endogenous contrast alternative to discriminate between healthy and infarcted myocardium [1-6].T1ρ times characterize relaxation in the rotating frame of reference induced by an RF pulse, the so-called spin-lock (SL) pulse [7]. Spin-lock preparations with variable durations are used to generate varying contrast and allow for T1ρ quantification.
While promising results were obtained at low field strength [5], its translation to higher field strengths is hindered by the susceptibility of the spin-lock preparation to B0 and B1+ field inhomogeneities. Adiabatic spin-lock modules have the potential to overcome these limitations and allow the use of T1ρ as an endogenous contrast agent in clinical practice [8-9]. Thus, in this work we sought to enable robust in-vivo T1ρ-mapping at 3T in a single breath-hold using adiabatic spin-lock preparations.
Methods
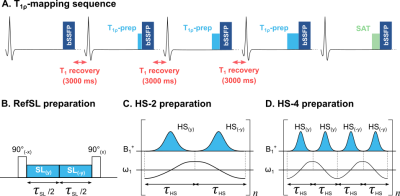
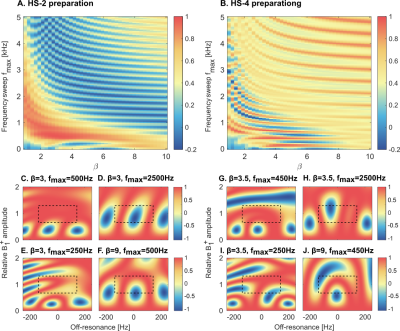
T1ρ-mapping was performed on a 3T scanner (Ingenia, Philips) with a breath-hold ECG-triggered single-shot bSSFP sequence (Fig1).A conventional spin-lock module (RefSL) was compared to adiabatic T1ρ preparations, obtained by concatenating 2 (HS-2) or 4 (HS-4) phase-cycled hyperbolic-secant adiabatic full-passage pulses (Fig1.b-d) [10]. Duration of each pulse was set by the total spin-lock block duration of 60ms (𝜏HS=30ms for HS-2, 𝜏HS=15ms for HS-4). Peak RF amplitude was set to the allowed maximum (B+1,max=13.5μT). Optimization for the remaining HS pulse parameters (β, fmax) was based on Bloch simulations. The final longitudinal magnetization Mz was used as a measure of preparation efficiency. Average Mz was computed for every combination of β=[1:0.25:10] and fmax=[0:50:5000]Hz over a design window of ±150Hz off-resonances and ±25% B+1 variations [11].
The sequence was tested on NiCl2-agar phantoms and in-vivo in one healthy volunteer (female, 21y/o). T1ρ maps were generated using a three-parameter model.
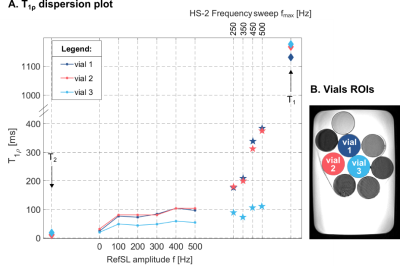
T1ρ dispersion was investigated in phantoms by varying the RefSL amplitude f=[0:100:500]Hz and HS-2 frequency sweep amplitude fmax=[250,350,500]Hz. T1ρ values were averaged over five repetitions for each spin-lock setting.
Results
Simulation results for HS-2 and HS-4 preparations averaged over the design window present periodic patterns across β and fmax values, with the best preparation efficiency for low to intermediate sweep amplitude and inversely-proportional β (Fig2a-b). Optimal values of {β, fmax} were chosen as {3, 500Hz} for HS-2 and {3.5, 450Hz} for HS-4, resulting in average residual magnetization Mz of 0.98 and 0.96, respectively. Examples of preparation efficiency over a range of B0 and B1+ inhomogeneities are shown for selected parameter combinations, demonstrating clusters of opposite polarity corrupting the magnetization preparation for sub-optimal parameters choice.Phantom magnitude images for different spin-lock durations and corresponding T1ρ-maps are shown for RefSL (f=500Hz), HS-2 and HS-4 (fmax=500Hz) (Fig3-a). HS-2 preparations yield the best results, with fewer artifacts than both HS-4 and RefSL, higher T1ρ contrast than non-adiabatic preparations (mean±std over vial 1-3=[384.95±40.04, 373.42±39.24, 108.19±9.87]ms HS-2, [104.96±87.25, 98.12±81.40, 54.64±48.71]ms RefSL), and less susceptibility to the B0 shim.
Non-adiabatic and adiabatic T1ρ dispersion results show consistent trends, with T1ρ values increasing with f and fmax, respectively (Fig4). Overall adiabatically-prepared sequences achieve longer T1ρ times than non-adiabatic pulses.
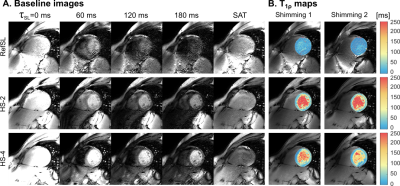
In-vivo T1ρ values (±std) in the myocardium were: [16.01±20.75, 148.13±54.08, 54.72±41.04]ms for RefSL, HS-2 and HS-4 preparations, respectively (Fig5). Adiabatically-prepared sequences show significantly higher myocardial/blood contrast than RefSL, while HS-4 presents residual artifacts for shimming 2.
Discussion
In this work we investigate the use of adiabatic preparations for B0 and B1+ resilient myocardial T1ρ-mapping at 3T. Our results show improved map quality for adiabatic preparations both in phantom and in-vivo, compared to the reference SL implementation. Adiabatically-prepared sequences demonstrate good blood myocardium contrast and retain image quality across different shim conditions. The effective field strength and orientation vary throughout the adiabatic spin-lock preparation. Thus, no uniform T1ρ time is sampled, but the relaxation changes throughout the preparation. Consequently, each adiabatic T1ρ-preparation probes a wider spectrum of frequencies through the adiabatic sweep, compared to mono-frequency conventional spin-lock. This may lead to different sensitivity profiles in pathological remodeling and its clinical value remains to be evaluated.Simulations were performed to optimize the adiabatic pulse parameters for 2 or 4 HS pulses with equal total duration. While 2 pulses were phase cycled, 4 pulses allow for a full MLEV scheme [10]. However, longer 30ms pulses in HS-2 show larger adiabatic regime regions (red areas in Fig.2a-b) compared with the HS-4 pulses (15ms each). Furthermore, the adiabatic regime is more clearly separated from the high-β/ high-fmax areas where very fast adiabatic sweeps do not induce any rotation and thus result in artificially high preparation efficiencies. Accordingly, in phantom experiments HS-2 preparations show reduced artifacts in the water bath. In-vivo, HS-2 maps are artifact-free for both shimming conditions. HS-4 maps, on the other hand, present residual off-resonance artifacts as well as more inhomogeneous T1ρ values across the myocardium. These results indicate that longer HS pulses, with slower frequency sweeps, achieve better performances.
Conclusions
Our results suggest that adiabatic T1ρ-mapping allows for robust in vivo quantification of myocardial spin-lock relaxation times at high field strengths. This paves the way for potential non-contrast imaging of myocardial fibrosis at 3T.Acknowledgements
Funding: 4TU, NWO Start-Up, ZonMW OffRoadReferences
- Witschey, W. R., Pilla, J. J., Ferrari, G., Koomalsingh, K., Haris, M., Hinmon, R., ... & Reddy, R. (2010). Rotating frame spin lattice relaxation in a swine model of chronic, left ventricular myocardial infarction. Magnetic resonance in medicine, 64(5), 1453-1460.
- Witschey, W. R., Zsido, G. A., Koomalsingh, K., Kondo, N., Minakawa, M., Shuto, T., ... & Gorman, R. C. (2012). In vivo chronic myocardial infarction characterization by spin locked cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance, 14(1), 1-9.
- Musthafa, H. S. N., Dragneva, G., Lottonen, L., Merentie, M., Petrov, L., Heikura, T., ... & Liimatainen, T. (2013). Longitudinal rotating frame relaxation time measurements in infarcted mouse myocardium in vivo. Magnetic resonance in medicine, 69(5), 1389-1395.
- van Oorschot, J. W., El Aidi, H., Gho, J. M., Froeling, M., Visser, F., Chamuleau, S. A., ... & Zwanenburg, J. J. (2014). Endogenous assessment of chronic myocardial infarction with T 1ρ-mapping in patients. Journal of Cardiovascular Magnetic Resonance, 16(1), 1-9.
- Berisha, S., Han, J., Shahid, M., Han, Y., & Witschey, W. R. (2016). Measurement of myocardial T1ρ with a motion corrected, parametric mapping sequence in humans. PLoS One, 11(3), e0151144.
- Stoffers, R. H., Madden, M., Shahid, M., Contijoch, F., Solomon, J., Pilla, J. J., ... & Witschey, W. R. (2017). Assessment of myocardial injury after reperfused infarction by T1ρ cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance, 19(1), 1-10.
- Martino, A. F., & Damadian, R. (1984). Improved discrimination of normal and malignant tissue using 1H NMR relaxation time measurements at 2.18 MHz. Physiological chemistry and physics and medical NMR, 16(1), 49-55.
- Michaeli, S., Sorce, D. J., Springer Jr, C. S., Ugurbil, K., & Garwood, M. (2006). T1ρ MRI contrast in the human brain: modulation of the longitudinal rotating frame relaxation shutter-speed during an adiabatic RF pulse. Journal of magnetic resonance, 181(1), 135-147.
- Mangia, S., Liimatainen, T., Garwood, M., & Michaeli, S. (2009). Rotating frame relaxation during adiabatic pulses vs. conventional spin lock: simulations and experimental results at 4 T. Magnetic resonance imaging, 27(8), 1074-1087.
- Garwood, M., & DelaBarre, L. (2001). The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. Journal of magnetic resonance, 153(2), 155-177.
- Kellman, P., Herzka, D. A., & Hansen, M. S. (2014). Adiabatic inversion pulses for myocardial T1 mapping. Magnetic resonance in medicine, 71(4), 1428-1434.
Figures