0271
Improved Myocardial Scar Visualization with Two-minute Free-breathing Joint Bright- and Black-blood Late Gadolinium Enhancement Imaging1IHU LIRYC, Electrophysiology and Heart Modeling Institute, Université de Bordeaux – INSERM U1045, Bordeaux, France, 2Department of Cardiovascular Imaging, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 3Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4INRIA, Université Côte d’Azur, Sophia Antipolis, France, 5Department of Cardiac Electrophysiology, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 6CIBM Center for Biomedical Imaging, Lausanne, Switzerland
Synopsis
Black-blood late gadolinium enhancement (LGE) techniques are increasingly being used to uncover myocardial scar patterns that may be otherwise confused with blood signal on conventional bright-blood LGE, especially when involving the subendocardium. With signal-attenuated blood and dark muscle representation, these techniques may however be hampered by a lack of anatomical information, making spatial localization of myocardial injuries a challenging task. Here we aim to assess the clinical performance of a novel LGE technique that combines both bright- and black-blood imaging in a single time-efficient free-breathing exam to obtain LGE images with unprecedented scar contrast and localization.
INTRODUCTION
Black-blood late gadolinium enhancement (LGE) techniques are increasingly being used to uncover myocardial scar patterns that may be otherwise confused with blood signal on conventional bright-blood LGE, especially when involving the subendocardium (1). With signal-attenuated blood and dark muscle representation, these techniques may however be hampered by a lack of anatomical information, making spatial localization of myocardial injuries a challenging task. Here we propose to combine both bright- and black-blood imaging in a single time-efficient free-breathing exam to obtain LGE images with unprecedented scar contrast and localization.METHODS
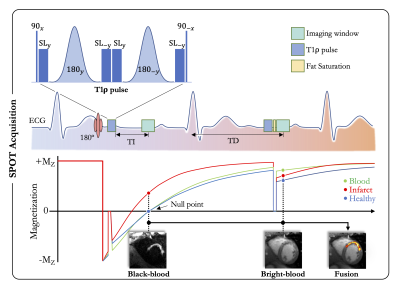
Acquisition: This Scar-specific imaging with Preserved myOcardial visualizaTion (SPOT) is a single-shot ECG-triggered 2D balanced steady-state free-precession (bSSFP) sequence that acquires bright- and black-blood LGE images in an interleaved fashion (Figure 1). In odd heartbeats, a 180° non-selective inversion pulse (pulse duration = 10ms) is followed by a T1ρ module (2) to generate black-blood contrast (for better scar visualization) whereas in even heartbeats only a T1ρ module is played out to generate bright-blood contrast (for better scar localization). The T1ρ module includes an initial 90° tip-down pulse along the x-axis to rotate the magnetization, followed by four spin-lock pulses with alternating phases (SL±y) and fixed duration, and by two adiabatic refocusing pulses (180±y). A final 90° tip-up pulse is then used to return the magnetization to the z-axis. A crusher gradient removes all residual transverse magnetization. For each slice, eight single-shot images (4 bright-blood and 4 black-blood images) were acquired in mid-diastole during 13 heartbeats (repetition time of two heartbeats to allow for magnetization recovery). Data were acquired during free-breathing with full complete ventricular coverage. Imaging parameters were: 10-20 slices, 4 signal averages, 1.4x1.4mm2 in-plane resolution, 8mm slice thickness, spin-lock frequency/duration=500Hz/27ms, FA=60°, GRAPPA x2, ~160ms acquisition window, TE/TR=1.2/2.8ms, bandwidth=870Hz/pixel. The inversion time was determined from a prior scout acquisition.Reconstruction: A non-rigid in-line motion correction algorithm was implemented on the scanner to register the 8 single-shot images to the same respiratory position, followed by image averaging. Three series of images were displayed on the scanner console: a bright-blood image, a black-blood counterpart, and a coloured fusion of both.
Experiments and analysis: 54 patients (44 males, age 18-82yo) with known ischemic heart disease underwent CMR at 1.5T (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany) with the reference breath-held 2D PSIR (3) and the proposed free-breathing SPOT 15min after injection of 0.2mmol/kg gadoteric acid (in random order). An experienced radiologist graded the overall image quality (1-nondiagnostic, 2-less than adequate, 3-adequate with moderate artifacts, 4-diagnostic image quality) and categorized LGE findings for each dataset as absent or definite with confidence or inconclusive. Acquisition times were recorded. Scores were compared using Wilcoxon matched-pairs signed-ranks test. The McNemar test was applied to compare the proportion of hyperenhanced lesions between the two techniques. All statistical tests were two-sided and values of P<0.05 indicated statistical significance.
RESULTS
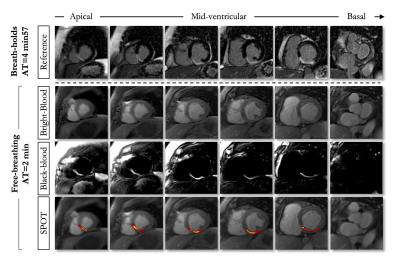
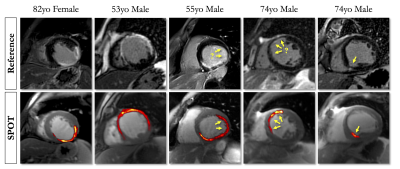
SPOT images were acquired with full ventricular coverage during free-breathing in 1min56s±30s (PSIR: 4min48s±1min16s, P<0.01). The homogenous suppression of blood and healthy myocardium on the black-blood images enabled a better visualization of myocardial scars on the SPOT images with remarkable spatial localization (Figures 2 and 3). Image quality was consistent among the two techniques (free-breathing SPOT: 3.7±0.5 vs. breath-held PSIR: 3.6±0.7, P=0.21). SPOT could identify 17% more hyperenhanced segments than PSIR (323 vs. 277, P<0.01). Seventeen patients (31%) had inconclusive LGE segments on PSIR. SPOT clearly showed hyperenhanced segments at these locations in 12/17 and could rule out areas of hyperenhancement in 5/17.CONCLUSION
SPOT can play a critical role in imaging patients with structural heart disease as it delivers images with unprecedented scar contrast embedded in a detailed heart anatomy and, in so doing, may provide opportunities for earlier detection and therapeutic management of structural heart disease.Acknowledgements
This research was supported by funding from the French National Research Agency under grant agreements ANR-21-CE17-0034-01, Equipex MUSIC ANR-11-EQPX-0030 and Programme d’Investissements d’Avenir ANR-10-IAHU04-LIRYC, and from the European Council under grant agreement ERC n715093. AB acknowledges a Lefoulon-Delalande Foundation fellowship administered by the Institute of France.References
1. Holtackers RJ, Van De Heyning CM, Chiribiri A, Wildberger JE, Botnar RM, Kooi ME. Dark-blood late gadolinium enhancement cardiovascular magnetic resonance for improved detection of subendocardial scar: a review of current techniques. J. Cardiovasc. Magn. Reson. 2021;23:1–18 doi: 10.1186/s12968-021-00777-6.
2. Bustin A, Toupin S, Sridi S, et al. Endogenous assessment of myocardial injury with single-shot model-based non-rigid motion-corrected T1 rho mapping. J. Cardiovasc. Magn. Reson. 2021;23, 119 doi:10.1186/s12968-021-00781-w.
3. Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn. Reson. Med. 2002;47:372–383 doi: 10.1002/mrm.10051.
Figures