0270
3D MRI atlases of congenital aortic arch anomalies and normal fetal heart: application to automated multi-label segmentation1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Department of Congenital Heart Disease, Evelina London Children’s Hospital, London, United Kingdom, 3Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
This work introduces the first black blood 3D T2w MRI atlases of the normal fetal heart and congenital aortic arch anomalies. The atlases were generated from 87 subjects from normal, CoA, RAA and DAA cohorts and also include multi-label segmentations of the major cardiovascular structures. We further evaluated the feasibility of using deep learning for automated multi-label vessel segmentation in 3D T2w motion-corrected MRI images of the fetal heart.
Introduction
The black blood contrast in T2w ssFSE fetal MRI provides superior visualisation of fetal cardiovascular anomalies [1]. Recently, application of slice-to-volume registration (SVR) motion correction tools [2] for reconstruction of high-resolution 3D T2w images of the fetal heart was shown to provide adjunct diagnostic information [3] and allow visualisation of the extracardiac vasculature using 3D models.At our institution, the main referral indications for 3D T2w fetal cardiac MRI (CMR) [3] include suspected coarctation of the aorta (CoA), right aortic arch (RAA) with aberrant left subclavian artery (ALSA) and suspected double aortic arch (DAA). However, despite the reported improved diagnostic performance of 3D fetal CMR [3,4], there is a lack of formalisation of the MRI appearance of fetal cardiovascular anatomy. Furthermore, currently, 3D semi-automatic segmentation of fetal vasculature [3] requires time-consuming manual refinement and is vulnerable to inter-observer bias.
In this work, we present the first 3D fetal MRI atlases defining normal and abnormal (suspected CoA, RAA+ALSA, DAA) fetal cardiovascular anatomy generated from 87 fetal CMR datasets along with the formalised multi-label parcellations of the major cardiovascular structures. This will contribute to standardisation of the 3D SVR-based diagnostic protocol and quantitative analysis. We also perform evaluation of the feasibility of deep learning for automated 3D multi-label fetal vessel segmentation.
Methods
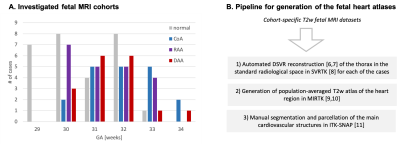
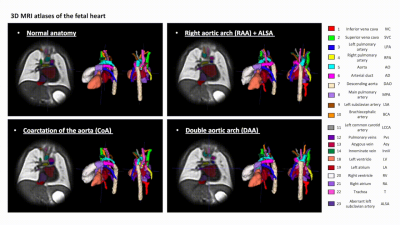
Datasets and preprocessing: The fetal MRI data include 87 datasets acquired under the iFIND [5] project at St. Thomas’s Hospital, London [REC: 14/LO/1806] and as a part of the fetal CMR service at Evelina London Children’s Hospital, with data use for this project subject to informed consent of the participants [REC: 07/H0707/105]. The cohorts (Fig.1A) include 29 fetuses without reported anomalies (“normal”) and 58 cases with postnatally confirmed anomaly (20 CoA, 21 RAA, 17 DAA). The inclusion criteria were high SNR, 29-34 weeks gestational age (GA) and absence of other cardiovascular anomalies. The acquisitions were performed on a 1.5T Philips Ingenia MRI system using torso receiver array and T2w ssFSE: TR=15000ms, TE=80ms, voxel size=1.25x1.25x2.5mm, slice thickness=2.5mm, spacing=1.25mm with 9-11 stacks. The 3D reconstructions of the fetal thorax (0.75mm isotropic resolution, standard radiological space) were generated using our new automated deformable SVR (DSVR) pipeline [6,7,8].Generation of 3D heart atlases with vessel segmentations: The 3D atlases for each of the groups (normal, CoA, RAA+ALSA, DAA) were generated using MIRTK atlas tool [9,10] with 0.6mm output isotropic resolution (Fig.1B). For each of the atlases, a clinician trained in fetal CMR (MVP) manually segmented 19 main cardiovascular structures in ITK-SNAP [11]. The chosen structures follow the clinical fetal CMR reporting protocol. The atlases are publicly available at [12].
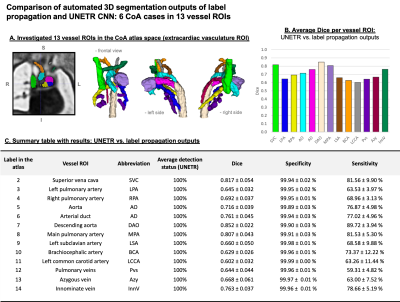
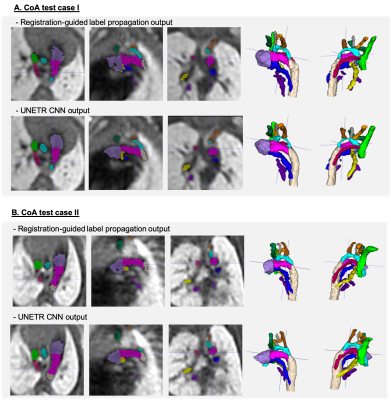
Automated segmentation of the fetal heart vessels: A pipeline for automated multi-label 3D segmentation of the cardiovascular structures in 3D DSVR MRI images was implemented in Pytorch MONAI [13] based on of UNETR transformer-based convolutional neural network (CNN) [14]. We used 39 cases for training and 4 for validation from normal and CoA cohorts. The preprocessing included cropping to the extracardiac vasculature ROI (e.g., Fig.4A) and affine registration to the atlas. The labels (13 vessels) were generated using MIRTK registration-guided cohort-specific atlas label propagation (LP). All labels were visually assessed and confirmed to be qualitatively acceptable. The training was performed for 50000 iterations with MONAI-based augmentation. The performance was tested on 6 CoA datasets vs. propagated labels in terms of the vessel detection status (visual assessment) and Dice.
Results and discussion
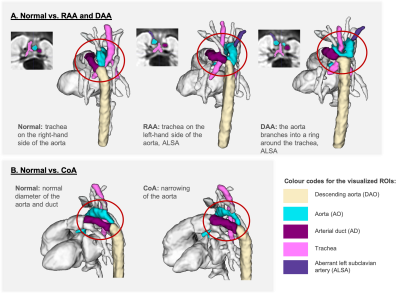
3D atlases: Fig.2 shows the generated 3D T2w atlases and the corresponding multi-label parcellations. The difference in the relative position of the trachea and the aortic arch(es) and aortic branching in the RAA and DAA atlases vs. the normal anatomy can be clearly seen in the corresponding 3D models (Fig.3A). [15]. The CoA atlas has a pronounced narrowing of the aortic arch (Fig.3B) [2,3]. Importantly, the CoA atlas represents an averaged state of all CoA types as the input cases had varying position and degree of arch narrowing. The anatomical accuracy of the atlases and the segmented structures were confirmed by a clinician with 4 years of fetal CMR experience (MVP).Automated vessel segmentation: The comparison between the automated outputs of the CNN vs. LP methods is summarised in Fig.4C. Similarly to the classical LP, UNETR correctly detected all vessels in all test CoA subjects (100%) as confirmed by high specificity. Notably, the registration propagated labels are prone to inconsistencies and cannot be considered as an absolute ground truth. This, along the small vessel size and the partial volume effect, contributed to the lower Dice and sensitivity values for certain vessels (e.g., BCA). The examples in Fig.5 demonstrate that while the global vessel anatomy looks similar, UNETR CNN segmentations have smoother boundaries and correct minor LP errors.
Conclusions
This work introduces the first set of 3D black blood MRI atlases of the normal and abnormal fetal cardiovascular anatomy along with segmentation of the major cardiovascular structures. This is a first step towards standardisation of the processing protocol for fetal cardiovascular anomalies using 3D motion-corrected T2w MRI. We also demonstrated the feasibility of using deep learning for multi-label vessel segmentation. Our future work will focus on optimisation of the CNN-based segmentation pipeline for a wider range of fetal cardiovascular anomalies.Acknowledgements
We thank everyone who was involved in acquisition and examination of the datasets and all participating mothers.
This work was supported by the Rosetrees Trust [A2725], the Wellcome/EPSRC Centre for Medical Engineering at King’s College London [WT 203148/Z/16/Z], the Wellcome Trust and EPSRC IEH award [102431] for the iFIND project, the NIHR Clinical Research Facility (CRF) at Guy’s and St Thomas’ and by the National Institute for Health Research Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London.The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. We thank everyone who was involved in acquisition and examination of the datasets and all participating mothers.
References
[1] Roy, C. W., van Amerom, J. F. P., Marini, D., Seed, M., & Macgowan, C. K. (2019). Fetal Cardiac MRI: A Review of Technical Advancements. Topics in Magnetic Resonance Imaging: TMRI, 28(5), 235–244.
[2] Kuklisova-Murgasova, M., Quaghebeur, G., Rutherford, M. A., Hajnal, J. v., & Schnabel, J. A. (2012). Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med Image Analysis, 16(8), 1550–1564.
[3] Lloyd, D. F. A., Pushparajah, K., Simpson, J. M., van Amerom, J. F., van Poppel, M. P. M., Schulz, A., Kainz, B., Deprez, M., Lohezic, M., Allsop, J., Mathur, S., Bellsham-Revell, H., Vigneswaran, T., Charakida, M., Miller, O., Zidere, V., Sharland, G., Rutherford, M., Hajnal, J., & Razavi, R. (2019). Three-dimensional visualisation of the fetal heart using prenatal MRI with motion-corrected slice-volume registration: a prospective, single-centre cohort study. The Lancet, 393(10181), 1619–1627.
[4] Lloyd, D. F. A., van Poppel, M. P. M., Pushparajah, K., Vigneswaran, T. v., Zidere, V., Steinweg, J., van Amerom, J. F. P., Roberts, T. A., Schulz, A., Charakida, M., Miller, O., Sharland, G., Rutherford, M., Hajnal, J. v., Simpson, J. M., & Razavi, R. (2021). Analysis of 3-Dimensional Arch Anatomy, Vascular Flow, and Postnatal Outcome in Cases of Suspected Coarctation of the Aorta Using Fetal Cardiac Magnetic Resonance Imaging. Circulation: Cardiovascular Imaging, 14(7), 583–593.
[5] “iFIND Project.” [Online]. Available: http://www.ifindproject.com/. [Accessed: 01-Nov-2021].
[6] Uus, A., Zhang, T., Jackson, L. H., Roberts, T. A., Rutherford, M. A., Hajnal, J. v, & Deprez, M. (2020). Deformable Slice-to-Volume Registration for Motion Correction of Fetal Body and Placenta MRI. IEEE Transactions on Medical Imaging, 39(9), 2750–2759.
[7] Uus, A., Grigorescu, I., van Poppel, M. P. M., Steinweg, J. K., Roberts, T. A., Rutherford, M. A., Hajnal, J. V., Lloyd, D. F. A., Pushparajah, K., & Deprez, M. (2021). Automated 3D reconstruction of the fetal thorax in the standard atlas space from motion-corrupted MRI stacks for 21-36 weeks GA range. BioRxiv, 2021.09.22.461335.
[8] “SVRTK: MIRTK based SVR package for fetal MRI.” [Online]. Available: https://github.com/SVRTK/. [Accessed: 01-Nov-2021].
[9] “MIRTK package.” [Online]. Available: https://github.com/BioMedIA/MIRTK/. [Accessed: 01-Nov-2021].
[10] Schuh, A., Murgasova, M., Makropoulos, A., Ledig, C., Counsell, S. J., Hajnal, J. v, Aljabar, P., & Rueckert, D. (2014). Construction of a 4D Brain Atlas and Growth Model using Diffeomorphic Registration. STIA MICCAI 2014.
[11] “ITK-SNAP tool.” [Online]. Available: http://www.itksnap.org/. [Accessed: 01-Nov-2021].
[12] “SVRTK fetal MRI data repository.” [Online]. Available: https://gin.g-node.org/SVRTK/. [Accessed: 01-Nov-2021].
[13] “MONAI framework.” [Online]. Available: https://github.com/Project-MONAI/MONAI/. [Accessed: 01-Nov-2021].
[14] Hatamizadeh, A., Tang, Y., Nath, V., Yang, D., Myronenko, A., Landman, B., Roth, H., & Xu, D. (2021). UNETR: Transformers for 3D Medical Image Segmentation. arXiv:2103.10504.
[15] van Poppel, M. P. M., Pushparajah, K., Lloyd, D. F. A., Razavi, R., Speggiorin, S., Nyman, A., Simpson, J. M., Zidere, V., & Vigneswaran, T. v. (2020). Insights from fetal cardiac magnetic resonance imaging in double aortic arch. Ultrasound in Obstetrics & Gynecology, 56(4), 636–639.
Figures