0269
Autonomic Function Regulates the Default-Mode Network in Rats1Biomedical Engineering, University of Michigan, ANN ARBOR, MI, United States, 2Electrical engineering and computer science, University of Michigan, ANN ARBOR, MI, United States
Synopsis
The default mode network (DMN) is central to cognition. Consistent across species, functional connectivity (FC) of DMN exhibits partly similar patterns across brain states, including wakefulness, sleep, sedated or anesthetized states. Regions in DMN are also involved in regulating visceral organs. It is likely that DMN facilitates visceral physiology to maintain homeostasis in states of unconsciousness. However, the evidence for the association between autonomic function and DMN is sparse and piecemeal. Here, we use fMRI in rat models to investigate how vagal nerve de-innervation and stimulation affect the DMN and its interaction with other regions related to autonomic function.
Purpose
The default mode network (DMN) [1] is central to cognition [2]. Its dysfunction is responsible for cognitive decline in many disease conditions [3,4]. Consistent across species [5], functional connectivity (FC) of DMN exhibits partly similar patterns across brain states, including wakefulness, sleep, sedated or anesthetized states [6,7]. Regions in DMN are also involved in regulating visceral organs [8]. It is likely that DMN facilitates visceral physiology to maintain homeostasis and necessitates life support in states of unconsciousness. However, the evidence for the association between autonomic function and DMN is sparse and piecemeal. This gap motivates us to use fMRI in rat models to investigate how vagal nerve de-innervation and stimulation affect the pattern of FC within DMN and its interaction with other regions related to autonomic function.Method
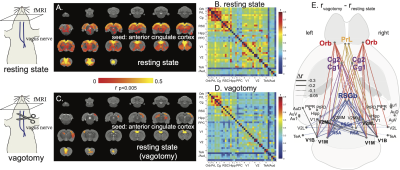
We performed fMRI in four groups of Sprague Dawley rats (n=5 per group). Group 1 and 2 underwent resting-state fMRI with intact cervical vagus nerves or cut bilaterally (i.e., vagotomy). Group 3 received repeated left cervical vagus nerve stimulation (VNS) with pulse width = 0.1ms, current 1mA, inter-pulse interval = 0.1s. Group 4 was the sham control to Group 3. All rats were anesthetized with continuous infusion of dexmedetomidine (SC, 0.015 mg/Kg/h) and inhalant isoflurane (0.1-0.5% mixed in O2) during fMRI. The fMRI scans used 2-D single-shot gradient-echo echo-planar imaging (EPI, TR=1s, TE=16.5ms, FA=60°, voxel size=0.5×0.5×1 mm3) in a 7-T small-animal MRI system (BioSpec 70/30, Bruker). The period of fMRI scans was >30mins in each rat. After preprocessing, we evaluated FC within and beyond DMN with seed-based correlations and correlations between anatomically defined brain parcels. The FC was measured in terms of the z score. The difference between groups was evaluated using a two-sample t-test to test the effects of vagotomy and VNS on FC associated with DMN.Results
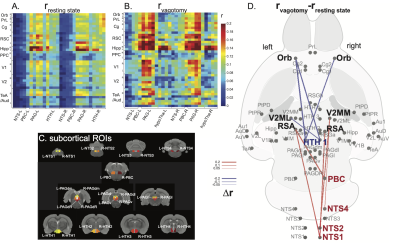
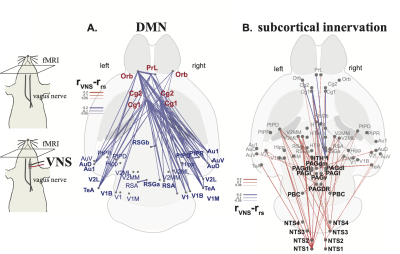
Correlations to the anterior cingulate cortex in rat brains showed a functional network analogous to DMN in human brains (Figure 1.a/b) [9]. This network depended on the vagus. When bilateral cervical vagotomy was applied to cause vagal de-innervation of visceral organs, FC within the DMN was largely reduced (Figure 1.c/d) with significant changes observable between the anterior and posterior DMN (Figure 1.e). We further examined whether this reduction was related to the subcortical nuclei that resided in the vagal ascending pathway, such as the nucleus tractus solitarius (NTS), parabrachial complex (PBC), periaqueductal grey (PAG), and hypothalamus (HTH). Bilateral vagotomy significantly increased FC between NTS/PBC and posterior DMN and decreased functional connectivity between HTH and anterior DMN (Figure 2). These results suggest that the vagus modulates DMN through subcortical nuclei involved in the central regulation of autonomic function. To further test the causal effects from the vagus, we applied VNS and evaluated the resulting changes in FC. VNS reduced FC within DMN, especially between its medial anterior regions and lateral posterior regions (Figure 3.a). VNS induced profound changes to the FC between regions in DMN and subcortical nuclei involved in autonomic control (Figure 3.b).Conclusion
Results shown herein demonstrate that vagus-mediated autonomic function may regulate the default mode network. The vagal signaling that allows the brain to monitor and regulate visceral function influences functional connectivity both within the default mode network and between the default mode network and subcortical nuclei involved in autonomic function. Direct vagal stimulation causes profound changes to the default mode network and its interaction with the autonomic ascending pathway in the brain. The relationship between autonomic function and the default mode network has implications for understanding the central and peripheral mechanisms for the co-occurrence of cognitive deficits and visceral diseases.Acknowledgements
The research is partly supported by NIH OD023847, OD030538, AT011665, DK131524.References
[1] Raichle, M.E., MacLeod, A.M., Snyder, A.Z., Powers, W.J., Gusnard, D.A. and Shulman, G.L., 2001. A default mode of brain function. Proceedings of the National Academy of Sciences, 98(2), pp.676-682.
[2] Margulies, D.S., Ghosh, S.S., Goulas, A., Falkiewicz, M., Huntenburg, J.M., Langs, G., Bezgin, G., Eickhoff, S.B., Castellanos, F.X., Petrides, M. and Jefferies, E., 2016. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proceedings of the National Academy of Sciences, 113(44), pp.12574-12579.
[3] Buckner, R.L., Andrews-Hanna, J.R. and Schacter, D.L., 2008. The brain's default network: anatomy, function, and relevance to disease.
[4] Sheline, Y.I., Barch, D.M., Price, J.L., Rundle, M.M., Vaishnavi, S.N., Snyder, A.Z., Mintun, M.A., Wang, S., Coalson, R.S. and Raichle, M.E., 2009. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences, 106(6), pp.1942-1947.
[5] Raichle, M.E., 2015. The brain's default mode network. Annual review of neuroscience, 38, pp.433-447.
[6] Greicius, M.D., Kiviniemi, V., Tervonen, O., Vainionpää, V., Alahuhta, S., Reiss, A.L. and Menon, V., 2008. Persistent default‐mode network connectivity during light sedation. Human brain mapping, 29(7), pp.839-847.
[7] Horovitz, S.G., Fukunaga, M., de Zwart, J.A., van Gelderen, P., Fulton, S.C., Balkin, T.J. and Duyn, J.H., 2008. Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG‐fMRI study. Human brain mapping, 29(6), pp.671-682.
[8] Bär, K.J., de la Cruz, F., Schumann, A. and Wagner, G., 2015. Relation of autonomic measures to the Default Mode Network. Autonomic Neuroscience: Basic and Clinical, 192, p.11.
[9] Lu, H., Zou, Q., Gu, H., Raichle, M.E., Stein, E.A. and Yang, Y., 2012. Rat brains also have a default mode network. Proceedings of the National Academy of Sciences, 109(10), pp.3979-3984.
Figures