0268
Alterations in intrinsic functional networks in squirrel monkey brain produced by dorsal column lesion of spinal cord using resting state fMRI1Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
The goal was to study how intrinsic functional networks within squirrel monkey brain undergo changes in connectivity after a dorsal column lesion (DCL) of the spinal cord. We used independent component analysis (ICA) to decompose whole brain fMRI data into spatially independent functional networks. Thirteen networks were identified, many of which resemble networks from human and macaque brain studies. Inter-network connectivity was computed before and after DCL of the cervical spinal cord. Changes in inter-network connectivity were not restricted to sensorimotor areas but spread over other cortical areas. Also, one of the connectivity increased significantly, possibly indicating compensation post lesion
Introduction
Spinal cord injury (SCI) results in motor and/or sensory dysfunction1,2. Over time, some functions recover spontaneously, while others remain permanently impaired. Plastic changes, including reactivation and reorganization in subcortical and cortical brain regions are believed to play crucial roles in the functional and behavioral recovery after SCI3. A dorsal column lesion (DCL) within the cervical cord is an appropriate model for the study of brain reorganization and functional recovery in non-human primates4. However, most previous investigations on this model have limited their observations to the organization of somatosensory cortex based on known anatomical connections2. There has not been any previous study on how whole brain functional networks are modified and undergo plastic changes after unilateral DCL of the spinal cord. We address this gap with two major objectives. Firstly, to identify and delineate resting state functional networks in normal squirrel monkey whole brain without any prior hypothesis of their natures. To achieve this, we use Independent Component Analysis (ICA) of resting state signals, a data-driven, hypothesis-free approach. Secondly, we quantified the changes in functional connectivity between the brain networks subsequent to a targeted DCL of the monkey cervical spinal cord.Methods
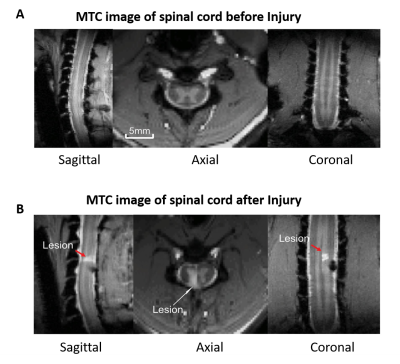
MRI scans were acquired on a Varian/Agilent 9.4T spectrometer from 19 normal (36 runs) and 4 DCL (14 runs) squirrel monkeys using a DOTY RF coil covering their whole brains. High resolution T2*W anatomical images were acquired (24 contiguous slices) along with resting state fMRI echo-planar imaging (EPI) data (TR/TE=1500/16 ms (2 shots), matrix size 64x64, voxel size: 1x1x1 mm3, 300 volumes each run) from the same geometry. Unilateral surgical transection was used to create a DCL at C5 level. Anatomical Magnetization Transfer Contrast (MTC) MRI images were also acquired from the spinal cord pre- and post-injury covering C3-C7 segments using a customized saddle coil. Standard data preprocessing was performed for whole brain EPI data which included corrections for motion and physiological signals (RETROICOR) and band pass filtering (0.01-0.1 Hz)5. This was followed by 2D registration to the squirrel monkey brain template (VALiDATe29 Atlas) using FSL in order to facilitate group level analyses6,7. Next, group spatial ICA was performed by temporal concatenation of all the EPI data from normal monkeys using GIFT software8, and fifteen spatially independent components were extracted from the whole brain. Thirteen components were identified to belong to cortical gray matter by visual inspection of each component’s spatial profile. Next functional connectivities between the thirteen identified networks were computed from both normal and injured monkeys by correlating (Pearson’s correlation r) the fMRI time courses between the voxels of those networks. Significant changes in inter-network connectivity post injury (t-test with multiple comparisons correction) were noted, and connectivity values from those networks were plotted before and after injury.Results
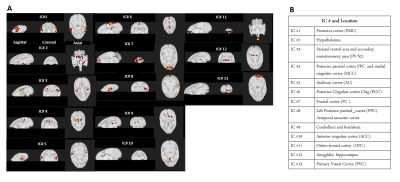
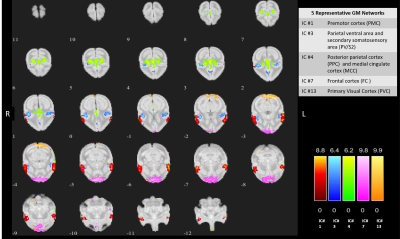
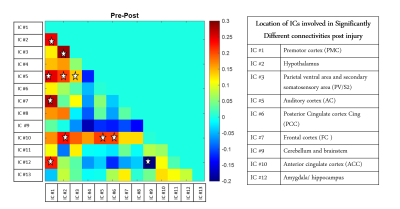
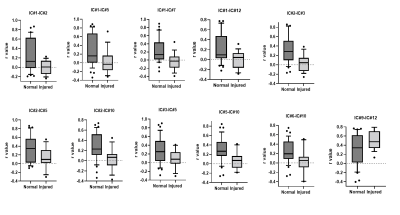
The thirteen spatially distinct cortical networks obtained from ICA of whole brain resting state fMRI data of normal monkeys are shown in Figure1A. The locations of the spatial components were identified using the squirrel monkey brain atlas and are shown in Figure1B. Many of these networks has been reported in previous studies on human and macaque brain. Figure2 shows the whole brain coverage of five representative networks. Figure3 shows MTC images of the cervical spinal cord pre- and post DCL. Spinal cord injury effects are visible and shown in Figure3B using arrows. Figure4 shows the differences between pre- and post-injury inter-network functional connectivity. Most of the connectivities reduced weeks after injury (positive values) while a small subset increased (negative value). The networks which are involved in significant changes (p<0.05) are shown in a table in Figure 4B. The inter-network connectivities that changed significantly are shown for pre- and post-injury monkeys using a box-plot (Figure5). While many of the connectivities decreased significantly (viz. between Premotor Cortex (IC#1) and Frontal Cortex (IC#7), between Posterior Cingulate Cortex (IC#6) and Anterior Cingulate Cortex (IC#10)), the inter-network connectivity between Cerebellum/brainstem (IC#9) and Amygdala/hippocampus (IC#12) increased significantly.Discussion and Conclusion
ICA of squirrel monkey whole brain revealed distinct, separable intrinsic functional networks, many of which have been reported previously in human and macaque brain studies9,10. Resting state fMRI detected significant changes in functional connectivity after a DCL of spinal cord demonstrating that injuries in the cord produce changes in network configurations in the brain. Changes due to a DCL were not restricted to sensorimotor areas but were also reflected in other cortical areas viz. Anterior and Posterior cingulate cortex (IC#10 and IC#6), Frontal cortex (IC#7) and Hypothalamus (IC#2). While connectivity strengths between some areas showed significant increase, most of the others exhibited significant decrease, indicating both compensation and disruption in connectivity in monkey brain post DCL of spinal cord. The information obtained about the network reorganization across functional regions post DCL will elucidate the specific roles that each area plays and provide knowledge that is critical for developing effective therapeutic and neuro-modulation strategies to promote recovery after SCI. Moreover, these results provide further validation that resting state fMRI detects baseline intrinsic architecture of brain networks and is sensitive to interventions that disrupt normal configurations. Future work will look into how the connectivities change longitudinally and whether they correlate with recovery of hand grasping behavior.Acknowledgements
This study is supported by NIH R01 NS078680 grant. Authors Li Min Chen and John C Gore share equal contribution.References
1. Finger S, Almli CR. Brain damage and neuroplasticity: mechanisms of recovery or development Brain Res. 1985
2. Yang PF, Qi HX, Kaas JH, Chen LM. Parallel functional reorganizations of somatosensory areas 3b and 1, and S2 following spinal cord injury in squirrel monkeys. J Neurosci. 2014.
3. Kaas JH, Qi HX, Burish MJ, Gharbawie OA, Onifer SM, Massey JM. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp Neurol. 2008
4. Chen LM, Qi HX, Kaas JH. Dynamic reorganization of digit representations in somatosensory cortex of nonhuman primates after spinal cord injury. J Neurosci. 2012.
5. Glover, G. H., Li, T. Q. & Ress, D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med.
6. Jenkinson, M. & Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001.
7. Schilling KG, Gao Y, Stepniewska I, et al. The VALiDATe29 MRI based multi-channel atlas of the squirrel monkey brain. Neuroinformatics. 2017.
8. Calhoun, V. D., Adali, T., Pearlson, G. D. & Pekar, J. J. Group ICA of functional MRI data: separability, stationarity, and inference. Proc. ICA.2001.
9. Zhang W, Jiang X, Zhang S, et al. Connectome-scale functional intrinsic connectivity networks in macaques. Neuroscience. 2017 10. Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006.
Figures