0266
Intercollicular interactions drive ipsilateral negative BOLD responses upon monaural auditory stimulation1Champalimaud Research, Champalimaud Centre for the Unknown, Lisboa, Portugal
Synopsis
Negative BOLD responses (NBRs) in rat Inferior Colliculus (IC) were recently observed upon monaural auditory stimulation, but their origins and importance remain poorly understood. Intercollicular communication is proposed as a prominent mechanism for auditory processing, including sound localization/lateralization & in gain control regulation. Here, we investigated intercollicular interaction via monoaural stimulation at 9.4T. Rats exhibited NBRs in the ipsilateral IC and positive BOLD responses (PBRs) in the contralteral IC. When the contralateral hemisphere was lesioned, the NBRs vanished in the ipsilateral IC. Our findings suggest that intercollicular interaction is essential for ipsilateral negative BOLD responses and for auditory processing.
Introduction
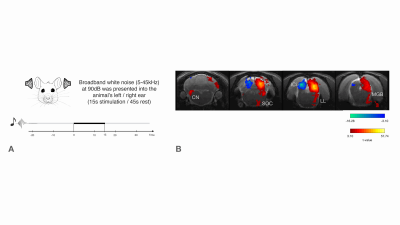
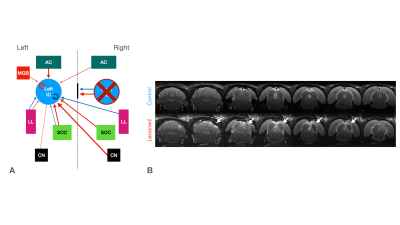
Functional MRI (fMRI) has been instrumental in characterizing the intact auditory pathway in humans1 and in preclinical imaging. Many critical features have been resolved, including tonotopy2,3, sound pressure level encoding4 or laterality5,6. In most studies, positive BOLD responses (PBRs) were observed along the different structures of the auditory pathway, contralaterally to the presented sound. We have recently discovered strong negative ipsilateral BOLD responses (NBRs) in IC upon monaural stimulation (Fig.1b)7, a critical junction in the auditory processing pathway. Yet, the origins of these NBRs remains poorly understood. Importantly, ICs do not operate in isolation, and the largest afferent source to each IC has been suggested to be their contralateral IC8,9, through excitation, inhibition, or a combination of both10. Therefore, we hypothesized that intercollicular interactions are responsible for this ipsilateral negative BOLD response (Fig.2a) and that they represent an important aspect of collicular processing11,12. We test this hypothesis using a lesion fMRI study in rats, at 9.4T, and show that indeed, intercollicular activity is sufficient and necessary for inducing NBRs, suggesting that activation and suppression feedback mechanisms play important roles in auditory processing.Methods
Animal experiments were preapproved by the institutional and national authorities and carried out according to European Directive 2010/63. Adult Long Evans rats (total n=12) were sedated with medetomidine13, while temperature and respiration rates were monitored and kept stable. Animals were divided into two groups, “Control” (n=6) and “Lesioned” (n=6). Controls consist of healthy animals, while Lesioned animals were previously subjected to the injection of Ibotenic Acid in the rightside IC. The Lesioned group was scanned 20-30 hours after surgery.MRI experiments: A 9.4T BioSpec scanner (Bruker, Karlsruhe, Germany) with an 86mm quadrature resonator for transmittance and a 4-element array cryoprobe (Bruker, Fallanden, Switzerland) for signal reception were used. Data were acquired under medical air (28%O2) with a GE-EPI sequence (TE/TR=14/1000ms, FOV=20x13mm2, in-plane resolution=250x250μm2, slice thickness=1mm, tacq=6min45s).
Auditory Stimulation: Monaural Broadband (5-45kHz) white noise at 90dB presented into the animal’s left/right ear (Fig.1a). Stimulation paradigm consisted of six repetitions of 15s stimulation and 45s rest.
IC Lesions: Anatomical scans of each animal were acquired and superimposed on a rat brain atlas14, where injection coordinates were determined. Lesions were performed unilaterally through a craniotomy by injecting ibotenic acid into the right IC. Post-surgery anatomical scans confirmed the location and extent of the lesions (Fig.2b).
Data analysis: Manual outlier correction; motion correction, slice-timing; smoothing (FWHM=0.250mm isotropic); mean-volume realignment and ROI analysis.
Results
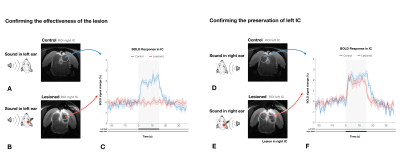
We first confirm that the lesion induced aberrations in sound processing by presenting sound to the rat’s left ear (Fig.3a and 3b). No activation in the contralateral IC was observed for the Lesioned group when auditory stimulation was presented, while the Control group exhibits the expected PBR in the contralateral (right) IC (Fig3.c).To ensure that the left collicular function remains intact (as potential spillover from the injections could affect that area) we presented the sound to the right ear (Fig.3d and 3e). In the contralateral (left, healthy on both groups) IC, similar responses between Lesioned and Control groups were observed, suggesting that activity remains intact (Fig. 3f).
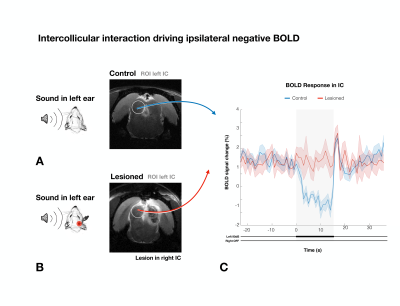
To study if intercollicular interactions drive ipsilateral NBRs, we presented sound to the left ear, looking at the left ipsilateral IC, (Fig.4). In the Lesioned group, ipsilateral IC NBR were effectively eliminated, with only a sharp positive signal at the end of the stimulation (also observed in the Control group).
Discussion
Our results reveal that auditory NBRs are driven by intercollicular interactions. In the Lesioned group, negative BOLD in ICi (ipsilateral IC) is effectively eliminated when ICc (contralateral IC) is lesioned, pointing to intercollicular inhibitory/excitatory interactions as a possible cause for this response. It is unlikely that the ICc lesions themselves affected the ICi (Fig.3f), suggesting that the contralateral (lesioned) ICc is responsible, at least in part, for the NBRs. Ipsilateral suppression of responses to contralateral stimulation has been previously suggested15, possibly through MGB feedback to the ipsilateral IC16,17, or intercollicular projections via the commissure of the IC11,12,18–21. Further studies focusing on the inactivation of these regions are crucial to understand the role of each individual structure in this NBR. Moreover, the role of each IC in binaural integration in rodents should be taken into consideration. Our findings pave the way to investigate brain-wide responses upon binaural stimulation.Interestingly, the ICi post stimulus PBR is preserved after ICc lesioning (Fig.5c), suggesting a different pathway is responsible for this sudden increase in signal, possibly a result of activity in auditory offset neurons, neurons directly responding to termination of sound22, as they have been found throughout most of the rodent auditory system23-25.
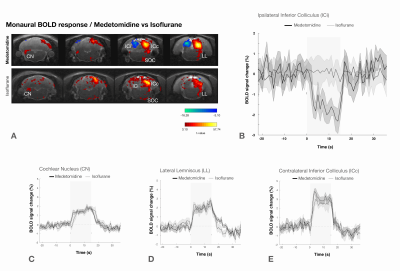
Previous studies did not detect negative BOLD under similar stimulation conditions2. We show that ipsilateral NBRs are abolished under isoflurane (Fig.5), which was used in3-5, underscoring that anesthesia can affect brain states and vascular dynamics26.
Conclusion
This work revealed how contralateral IC lesions affect ipsilateral IC responses, effectively abolishing ipsilateral NBRs upon monaural stimulation, suggesting strong intercollicular inhibitory/excitatory interactions.Acknowledgements
No acknowledgement found.References
1. Wessinger CM, Buonocore MH, Kussmaul CL, Mangun GR. Tonotopy in human auditory cortex examined with functional magnetic resonance imaging. Hum. Brain Mapp. 1997 doi: 10.1002/(SICI)1097-0193(1997)5:1<18::AID-HBM3>3.0.CO;2-Q.
2. Cheung MM, Lau C, Zhou IY, et al. BOLD fMRI investigation of the rat auditory pathway and tonotopic organization. Neuroimage 2012 doi: 10.1016/j.neuroimage.2012.01.087.
3. Cheung MM, Lau C, Zhou IY, et al. High fidelity tonotopic mapping using swept source functional magnetic resonance imaging. Neuroimage 2012 doi: 10.1016/j.neuroimage.2012.03.031.
4. Zhang JW, Lau C, Cheng JS, et al. Functional magnetic resonance imaging of sound pressure level encoding in the rat central auditory system. Neuroimage 2013 doi: 10.1016/j.neuroimage.2012.09.069.
5. Lau C, Zhang JW, Cheng JS, Zhou IY, Cheung MM, Wu EX. Noninvasive fMRI Investigation of Interaural Level Difference Processing in the Rat Auditory Subcortex. PLoS One 2013 doi: 10.1371/journal.pone.0070706.
6. Blazquez Freches G, Chavarrias C, Shemesh N. BOLD-fMRI in the mouse auditory pathway. Neuroimage 2018 doi: 10.1016/j.neuroimage.2017.10.027.
7. Frederico Severo, Rita Gil NS. Monoaural auditory stimulation induces negative BOLD along the rat auditory pathway. In: 30th ISMRM & SMRT Annual Meeting & Exhibition. ; 2021.
8. Games KD, Winer JA. Layer V in rat auditory cortex: Projections to the inferior colliculus and contralateral cortex. Hear. Res. 1988 doi: 10.1016/0378-5955(88)90047-0.
9. Moore DR. Auditory brainstem of the ferret: Sources of projections to the inferior colliculus. J. Comp. Neurol. 1988 doi: 10.1002/cne.902690303.
10. Keine C, Rübsamen R, Englitz B. Inhibition in the auditory brainstem enhances signal representation and regulates gain in complex acoustic environments. Elife 2016 doi: 10.7554/eLife.19295.
11. Adams JC. Crossed and descending projections to the inferior colliculus. Neurosci. Lett. 1980 doi: 10.1016/0304-3940(80)90246-3.
12. Saldaña E, Merchań MA. Intrinsic and commissural connections of the rat inferior colliculus. J. Comp. Neurol. 1992 doi: 10.1002/cne.903190308.
13. Weber R, Ramos-Cabrer P, Wiedermann D, Van Camp N, Hoehn M. A fully noninvasive and robust experimental protocol for longitudinal fMRI studies in the rat. Neuroimage 2006 doi: 10.1016/j.neuroimage.2005.08.028.
14. Paxinos G, Charles Watson. The Rat Brain in Stereotaxic Coordinates Sixth Edition.; 2007.
15. Tsytsarev V, Tanaka S. Intrinsic optical signals from rat primary auditory cortex in response to sound stimuli presented to contralateral, ipsilateral and bilateral ears. Neuroreport 2002 doi: 10.1097/00001756-200209160-00019.
16. Kuwabara N, Zook JM. Geniculo-collicular descending projections in the gerbil. Brain Res. 2000 doi: 10.1016/S0006-8993(00)02695-0.
17. Caicedo A, Herbert H. Topography of descending projections from the inferior colliculus to auditory brainstem nuclei in the rat. J. Comp. Neurol. 1993 doi: 10.1002/cne.903280305.
18. Ayala YA, Malmierca Dr. MS. Stimulus-specific adaptation and deviance detection in the inferior colliculus. Front. Neural Circuits 2012 doi: 10.3389/fncir.2012.00089.
19. Ito T, Bishop DC, Oliver DL. Two classes of GABAergic neurons in the inferior colliculus. J. Neurosci. 2009 doi: 10.1523/JNEUROSCI.3454-09.2009.
20. Moore DR, Kotak VC, Sanes DH. Commissural and lemniscal synaptic input to the gerbil inferior colliculus. J. Neurophysiol. 1998 doi: 10.1152/jn.1998.80.5.2229.
21. Reetz G, Ehret G. Inputs from three brainstem sources to identified neurons of the mouse inferior colliculus slice. Brain Res. 1999 doi: 10.1016/S0006-8993(98)01230-X.
22. Kopp-Scheinpflug C, Sinclair JL, Linden JF. When Sound Stops: Offset Responses in the Auditory System. Trends Neurosci. 2018 doi: 10.1016/j.tins.2018.08.009.
23. Xie R, Gittelman JX, Pollak GD. Rethinking tuning: In vivo whole-cell recordings of the inferior colliculus in awake bats. J. Neurosci. 2007 doi: 10.1523/JNEUROSCI.2865-07.2007.
24. Phillips DP, Hall SE, Boehnke SE. Central auditory onset responses, and temporal asymmetries in auditory perception. Hear. Res. 2002 doi: 10.1016/S0378-5955(02)00393-3.
25. Takahashi H, Nakao M, Kaga K. Cortical mapping of auditory-evoked offset responses in rats. Neuroreport 2004 doi: 10.1097/01.wnr.0000134848.63755.5c.
26. Paasonen J, Stenroos P, Salo RA, Kiviniemi V, Gröhn O. Functional connectivity under six anesthesia protocols and the awake condition in rat brain. Neuroimage 2018 doi: 10.1016/j.neuroimage.2018.01.014.
Figures