0258
Evaluation of Single-shot EPI with Sub-millimeter Resolution fMRI on the Next-Generation 7T brain scanner.1University of California, Berkeley, CA, United States, 2Advanced MRI Technologies, Sebastopol, CA, United States, 3Radiology, University of California, San Francisco, CA, United States, 4San Francisco Veteran Affairs Health Care System, San Francisco, CA, United States, 5Siemens Medical Solutions, Malvern, PA, United States, 6Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 7Harvard-MIT Health Sciences and Technology, MIT, Cambridge, MA, United States, 8Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 9Department of Radiology, Stanford University, Stanford, CA, United States, 10Department of Electrical Engineering, Stanford University, Stanford, CA, United States, 11Imaging Centre of Excellence, University of Glasgow, Glasgow, United Kingdom, 12MR CoilTech Limited, Glasgow, United Kingdom, 13Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
The newly developed “Impulse” head gradient (200 mT/m Gmax, 900 T/m/s) on the NexGen 7T scanner has been specifically designed to allow for high-resolution single-shot EPI with minimal distortions and blurring by minimizing echo spacing. In conjunction with the increased SNR and reduced g-factor noise afforded by the 96 channel receive array coil, mesoscale fMRI at 0.45mm and 0.6mm isotropic resolutions can be robustly achieved in human subjects.
Purpose
The Next Generation (NexGen) 7T MRI scanner1 was specifically designed to achieve higher resolution brain imaging2, with a novel head gradient coil, a novel 128-channel receive system (Rx) and 16-channel transmit system (pTx). Here, GE-EPI pulse sequences are optimized on the NexGen 7T scanner and evaluated to achieve higher spatial resolutions for higher granularity in fMRI research.Methods
The gradients and other hardware systems on the NexGen 7T scanner were designed and built under a BRAIN Initiative project. The PNS-optimized asymmetric head gradient coil (“Impulse” Siemens Healthcare), achieves Gmax of 200mT/m and slew rate (SR) of 900T/m/s1,3. The scanner is equipped with 16-channel pTx coil and a 96-channel Rx array4. The EPI sequence incorporated Dual Polarity GRAPPA (DPG) image reconstruction to reduce ghosting artifacts5. EPI data for comparison were also collected on a conventional 7T scanner (MAGNETOM Terra) equipped with a whole-body gradient (Gmax 70 mT/m, SR 200 T/m/s) and on the NexGen 7T by reducing the gradient strength and slew rate to “XR” gradients (80 mT/m, 200 T/m/s).For 0.45mm resolution fMRI, eight 3.75-min runs of flickering checkerboard stimuli were acquired using PsychoPy with a 7T-compatible video projector (VPixx Technologies). Images were retro-reconstructed with POCS. For 0.6mm isotropic fMRI four 5-min runs were acquired. Both protocols used TR of 3s. Functional analyses were performed with an afni_proc.py script modified for high-resolution data. No smoothing was applied and t-statistics were thresholded at p<0.01, spatially and temporal autocorrelation corrected. Temporal SNR (tSNR) analyses at different echo spacings and partial Fourier (PF) factors were performed using 30 repetitions of resting-state acquisitions. Blurring on the phase encoding axis was measured using AFNI’s 3dFWHMx.
Several hardware and integration challenges arose while testing EPI that had to be solved:
(1) The acquisition computer had to be modified to handle larger data acquired with the 128-channel receiver that scales drastically with the larger matrix sizes.
(2) Eddy current induced heating of the external RF shield of the Tx coil (MR CoilTech) and spike artifacts in the EPI acquisitions were solved with on-site work
(3) Mechanical resonances were encountered that led to the head gradient coil’s anterior support structure to develop cracks, which was overcome initially by excluding specific echo spacings and will be completely fixed by a new support structure designed, fabricated and tested by Siemens Healthcare.
(4) Ghosting artifacts were greatly reduced by improved calculation of short term eddy current compensation.
Results and Discussion
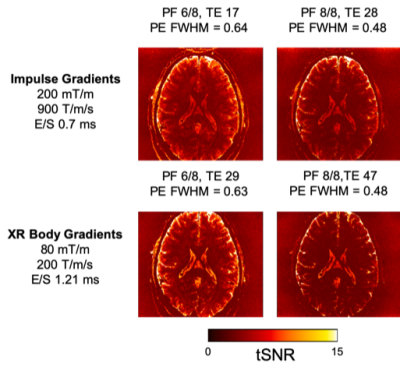
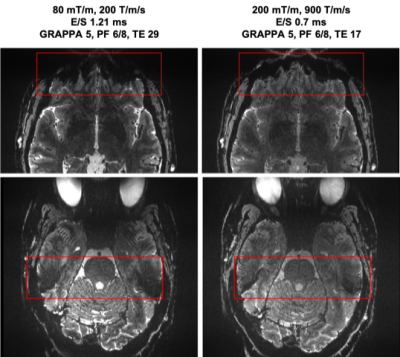
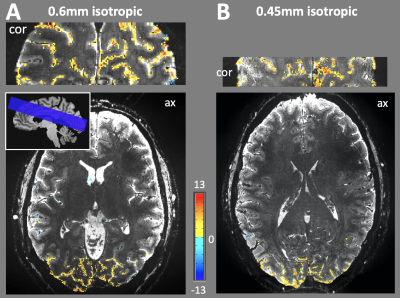
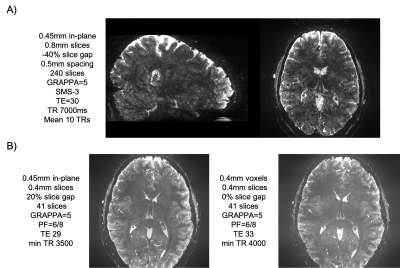
Several available techniques impact EPI resolution including partial Fourier6, parallel imaging7 and SMS8-10 while all necessarily elicit varying gains and penalty. Figure 1 demonstrates the TE values that can be achieved on the NexGen system compared to conventional 7T system’s gradient amplitudes and slew rates. For a given acceleration factor (e.g., GRAPPA=5), the NexGen gradients can achieve the same TE as conventional system/gradients without requiring the use of partial Fourier imaging that can introduce blurring on the phase encode axis2,6, reducing effective resolution, even with the use of advanced partial-Fourier reconstruction methods such as POCS (which reduced the measured PE FWHM from 0.63 to 0.54 for the PF 6/8 data at nominal 0.6mm resolution). Furthermore, to achieve the same effective resolution on conventional systems either requires additional acceleration or longer echo times, both leading to a reduction in SNR and CNR. In addition, the shorter echo spacings achievable with the “Impulse” gradients reduce geometric distortions and susceptibility dropout in EPI data (Figure 2). The shorter echo spacing also allows for less GRAPPA acceleration and/or partial Fourier imaging, leading to increases in SNR and effective resolution. Example fMRI activation using single-shot EPI without PF imaging are shown in Figure 3A, where activations follow the gray matter ribbon in visual cortex. The TE can be further reduced on the NexGen system by using RL rather than AP phase encoding for reduced FOV, normally limited by PNS on conventional whole-body gradient systems.The advantages of the NexGen 7T become especially apparent at ultra-high resolutions, where echo spacings on conventional systems must be so long that single-shot EPI is not practical, even at high accelerations (Table 1). fMRI using 0.45-mm in-plane single-shot EPI are shown in Figure 3B and at this resolution the cortical ribbon will be well sampled to resolve laminar activity. By increasing both accelerations, SMS factors in conjunction with in-plane acceleration, and increasing SNR by using thicker, dithered slices (SLIDER)2,11, full brain 0.45 mm in-plane imaging was achieved with TR of 7000 and TE of 30 ms (Figure 4A). Increasing in-plane resolution from 0.45-mm to 0.4mm shows only a slight increase in TE and TR, indicating 0.4-mm single-shot EPI could successfully be used for mesoscale fMRI (Figure 4B).
Summary and Conclusion
In summary, the cumulative effects of the stronger, faster gradients and high-density receiver arrays allow for shorter TEs, less distortion and lower g-factor noise for higher acceleration in ultra-high resolution functional imaging (0.6 mm and 0.45 mm) with larger FOVs and greater slice coverage than with the conventional whole-body gradient. In conclusion, the NexGen 7T scanner performs single-shot EPI at extremely high spatial resolution, normally not achievable with conventional 7T systems, at the scale of cortical layers and columns.Acknowledgements
Research supported by the BRAIN Initiative (NIH grants R0-MH111444 and U01-EB025162), and NIH R44-MH129278References
1. Feinberg DA, Dietz P, Liu C, Setsompop K, Mukherjee P, Wald LL, Vu AT, Beckett AJS, Insua IG, Schröder M, Stocker S, Bell PH, Rummert E, and Davids M “Design and Development of a Next-Generation 7T human brain scanner with high-performance gradient coil and dense RF arrays.” ISMRM 2021
2. Feinberg DA, Vu AT and Beckett AJS “Pushing the limits of ultra-high resolution human brain imaging with SMS-EPI demonstrated for columnar level fMRI” Neuroimage 2018
3. Davids M, Dietz P, Ruyters G, Roesler M, Klein V, Guerin B, Feinberg DA, and Wald LL “PNS optimization of a high-performance asymmetric gradient coil for head imaging” ISMRM 2021
4. Gunamony S, Müller R, McElhinney P, Williams SN, Groß-Weege N, Weiskopf N, Möller HE, and Feinberg DA “A 16-channel transmit 96-channel receive head coil for NexGen 7T scanner” ISMRM 2021
5. Hoge WS, Setsompop K, Polimeni JR “Dual-polarity slice-GRAPPA for concurrent ghost correction and slice separation in simultaneous multi-slice EPI” MRM 2018
6. Feinberg DA, Hale JD, Watts JC, Kaufman L, Mark A “Halving MR imaging time by conjugation: demonstration at 3.5 kG” Radiology 1986
7. Pruessmann JP, Weiger M, Scheidegger MB and Boesiger P “SENSE: sensitivity encoding for fast MRI” MRM 1999
8. Moeller S, Yacoub E, Olman CA, Auerbach A, Strupp J, Harel N, Uğurbil K “Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI”. MRM 2010
9. Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF , Miller KL, Ugurbil K and,Yacoub E “Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging” PLoS One 2010
10. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ and Wald LL “Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty” MRM 2012
11. Vu AT, Beckett AJS, Setsompop K and Feinberg DA “Evaluation of SLIce Dithered Enhanced Resolution Simultaneous MultiSlice (SLIDER-SMS) for human fMRI” Neuroimage 2018
Figures