0254
Revealing diffusion time-dependence and exchange effect in the in vivo human brain gray matter by using high gradient diffusion MRI1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Center of Functionally Integrative Neuroscience (CFIN) and MINDLab, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark, 4Department of Physics and Astronomy, Aarhus University, Aarhus, Denmark
Synopsis
The characteristic signal time-dependence in the brain gray matter indicates a significant exchange effect, modeled by an extension of the Kärger exchange model. Here we perform in vivo diffusion measurements with varying time scales (21-40 ms) up to high b-values (≤22 ms/µm2) in a healthy subject on an HCP scanner, and observe the signal time-dependence in frontal lobe, temporal lobe, and limbic system. The estimated residence time is 2-7 ms in the brain gray matter, suggesting that diffusion in neurite and extra-cellular space is coarse-grained at clinical diffusion time scale.
Introduction
Diffusion MRI (dMRI) is sensitive to the tissue microstructure, offering an estimate of cell dimensions in the brain white/gray matter (WM/GM) [1-5]. In particular, at sufficiently strong diffusion weightings (b-values), the extra-cellular water signal is exponentially suppressed, and the remaining intra-cellular signal can be used to quantify the cell features [6-7]. The infinitely thin fiber (stick) yields the characteristic $$$1/\sqrt{b}$$$ scaling of the directionally/powder-averaged dMRI signal, observed in vivo in the human brain WM up to $$$b$$$≤10 ms/μm2 [5-7], but not in GM potentially due to exchange [4-5]. By varying diffusion time scales at high b-values up to 10/100 ms/µm2 in the in vivo/ex vivo mouse brains [8-9], the observed signal time-dependence cannot be explained by the non-exchanging multi-compartmental Gaussian model. Instead, the signal time-dependence indicates a significant exchange effect, modeled by an extension of the Kärger model [10] with two exchanging anisotropic and isotropic Gaussian components, respectively [8-9]. Here, we performed in vivo diffusion measurements with varying time scales up to high b-values in the human brain GM, and fitted the anisotropic Kärger model to powder-averaged signals, enabling estimation of the intra-neurite residence time scale.Methods
Anisotropic Kärger modelThe dMRI signal in GM is assumed to be contributed by two exchanging Gaussian compartments, including an anisotropic "stick"-like compartment for neurites and an isotropic compartment for the extra-cellular space [8-9]. In ref. [9], this model is referred as the Standard Model with EXchange (SMEX) with 4 tissue parameters: neurite density $$$f_n$$$, intra-neurite diffusivity $$$D_n$$$, extra-cellular diffusivity $$$D_e$$$, and exchange rate $$$r_n$$$ (residence time $$$\tau_n=1/r_n$$$).
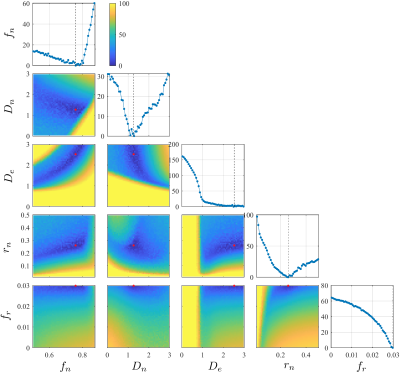
To account for the remaining noise floor due to nonlinear transformations (POCS, distortion corrections), a constant term $$$f_r$$$ is added. To stabilize the fitting, initial values are chosen based on the landscape of cost/energy function explored in the parameter space by using a neural network. A typical parameter landscape is in Fig. 1. Other fitting details are in ref. [9].
In vivo MRI
One healthy subject (26yo, M) underwent imaging on an HCP Siemens Skyra 3T scanner (maximal gradient=300 mT/m, slew rate=200 T/m/s) with a home-built 32-channel head coil [11] after providing consents. The dMRI measurements using monopolar pulsed-gradient spin-echo sequence were performed with 3 sequences:
1) $$$\Delta/\delta$$$=21/10 ms, $$$b$$$=[1, 3, 5.3, 7, 9.7] ms/µm2.
2) $$$\Delta/\delta$$$=30/10 ms, $$$b$$$=[1, 3, 5.3, 7, 9.8, 14.5] ms/µm2.
3) $$$\Delta/\delta$$$=40/10 ms, $$$b$$$=[1, 3, 5.3, 7, 9.8, 14.5, 21.9] ms/µm2.
In the above protocol, we applied gradients ≤293 mT/m. For each b-shell, we obtained 4 b=0 images and 64 DWIs for each b-shell. Resolution=(2 mm)3, FOV=220×220 mm2, 70 slices, TE/TR=67/4200 ms, PF=6/8 reconstructed with POCS, GRAPPA=2, SMS=2, total scan time=82 mins.
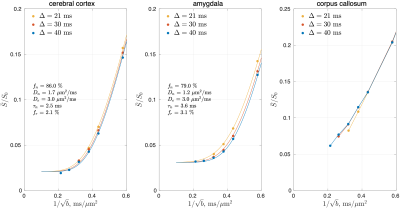
The shot-to-shot phase variation in each complex-valued DWI was eliminated [12], and the obtained real-valued DWIs were processed using DESIGNER pipeline except denoising and Rician bias correction [13]. Powder-averaged signals were obtained by averaging DWIs over directions at each time point and b-shell. SMEX was fitted to powder-averaged signals to estimate tissue parameters in each cerebral GM region-of-interest (ROI), segmented using FreeSurfer [14]. The signal in a representative WM ROI (corpus callosum) was also shown for reference.
To statistically decide the presence of signal time-dependence, a one-sided Wilcoxon signed-rank test is applied in each ROI, with a null hypothesis that powder-averaged signals do not decrease with time at each b-value. The significance level is 0.01.
Results
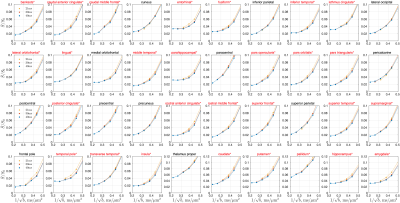
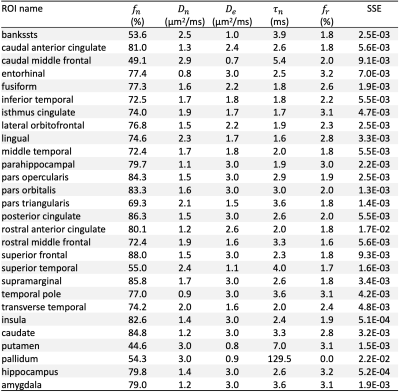
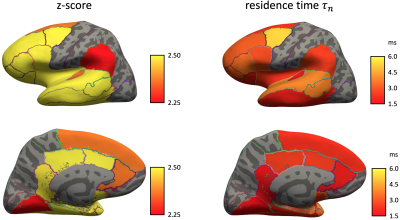
In cerebral cortex and amygdala, powder-averaged signals decrease with time at each b-value, indicating an observable exchange effect in GM (Fig. 2). Fitting the exchange model (SMEX) yields an estimate of residence time $$$\tau_n\approx$$$2.5-3.6 ms, suggesting a fast exchange regime in GM. In contrast, in corpus callosum, powder-averaged signals increase with time at high b-values, suggesting the applicability of non-exchanging non-Gaussian models [15,16] in WM.The signal decreasing with time at each b-value is also observed in many GM ROIs (Fig. 3). Interestingly, this characteristic signal time-dependence is mainly observed in the frontal lobe, temporal lobe and limbic system. Fitting SMEX to in vivo data in these ROIs yields an estimate of residence time $$$\tau_n\approx$$$2-7 ms (Fig. 4), again suggesting a fast exchange regime in GM. Similar results are also shown on the inflated GM surface (Fig. 5).
Discussion and conclusions
Significant signal time-dependence is in vivo observed at high b-values in the brain GM, especially in the frontal lobe, temporal lobe and limbic system. This unique functional form characterizes the water exchange (rather than soma) as the dominant mechanism for time-dependence in GM [8-9]. The estimated residence time is 2-7 ms in GM, faster than values (10-5000 ms) reported in previous studies [5,17-19]. The observed fast exchange in GM suggests that diffusion in intra-neurite space and extra-cellular space is coarse-grained at clinical diffusion time, and the two spaces can be modeled as a single Gaussian component with negligible higher order cumulants at long time. This potentially suggests a MINImal model in GRAy Matter (MiniGram) with 4 parameters ($$$D_e^\parallel,D_e^\perp,R_s,f_s$$$), consisting of an anisotropic Gaussian component and a restricted spherical compartment (soma), facilitating the estimation of soma density and size.Acknowledgements
We would like to thank Dmitry Novikov for the discussion of theory. Research was supported by the Office of the Director of the NIH under award DP5 OD031854, and by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the NIH under award U01 EB026996.
SJ acknowledges financial support for a sabbatical at NYU from Aarhus University Research Foundation (AUFF), the Lundbeck Foundation (R291-2017-4375), and Augustinus Fonden (18-1456). SJ and JO are also supported by the Danish National Research Foundation (CFIN), and the Danish Ministry of Science, Innovation, and Ed- ucation (MINDLab). Additionally, JO is supported by the VELUX Foundation (ARCADIA, grant no. 00015963).
References
1. Alexander, D. C., Hubbard, P. L., Hall, M. G., Moore, E. A., Ptito, M., Parker, G. J., & Dyrby, T. B. (2010). Orientationally invariant indices of axon diameter and density from diffusion MRI. Neuroimage, 52(4), 1374-1389.
2. Assaf, Y., Blumenfeld‐Katzir, T., Yovel, Y., & Basser, P. J. (2008). AxCaliber: a method for measuring axon diameter distribution from diffusion MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 59(6), 1347-1354.
3. Huang, S. Y., Tian, Q., Fan, Q., Witzel, T., Wichtmann, B., McNab, J. A., ... & Nummenmaa, A. (2020). High-gradient diffusion MRI reveals distinct estimates of axon diameter index within different white matter tracts in the in vivo human brain. Brain Structure and Function, 225(4), 1277-1291.
4. Palombo, M., Ianus, A., Guerreri, M., Nunes, D., Alexander, D. C., Shemesh, N., & Zhang, H. (2020). SANDI: a compartment-based model for non-invasive apparent soma and neurite imaging by diffusion MRI. NeuroImage, 215, 116835.
5. Veraart, J., Nunes, D., Rudrapatna, U., Fieremans, E., Jones, D. K., Novikov, D. S., & Shemesh, N. (2020). Noninvasive quantification of axon radii using diffusion MRI. Elife, 9, e49855.
6. McKinnon, E. T., Jensen, J. H., Glenn, G. R., & Helpern, J. A. (2017). Dependence on b-value of the direction-averaged diffusion-weighted imaging signal in brain. Magnetic resonance imaging, 36, 121-127.
7. Veraart, J., Fieremans, E., & Novikov, D. S. (2019). On the scaling behavior of water diffusion in human brain white matter. NeuroImage, 185, 379-387.
8. Jelescu, I. O., de Skowronski, A., Palombo, M., & Novikov, D. S. (2021). Neurite Exchange Imaging (NEXI): A minimal model of diffusion in gray matter with inter-compartment water exchange. arXiv preprint arXiv:2108.06121.
9. Olesen, J. L., Østergaard, L., Shemesh, N., & Jespersen, S. N. (2021). Diffusion time dependence, power-law scaling, and exchange in gray matter. arXiv preprint arXiv:2108.09983.
10. Fieremans, E., Novikov, D. S., Jensen, J. H., & Helpern, J. A. (2010). Monte Carlo study of a two‐compartment exchange model of diffusion. NMR in Biomedicine, 23(7), 711-724.
11. Stockmann, J. P., Witzel, T., Keil, B., Polimeni, J. R., Mareyam, A., LaPierre, C., ... & Wald, L. L. (2016). A 32‐channel combined RF and B0 shim array for 3T brain imaging. Magnetic resonance in medicine, 75(1), 441-451.
12. Eichner, C., Cauley, S. F., Cohen-Adad, J., Möller, H. E., Turner, R., Setsompop, K., & Wald, L. L. (2015). Real diffusion-weighted MRI enabling true signal averaging and increased diffusion contrast. NeuroImage, 122, 373-384.
13. Ades-Aron, B., Veraart, J., Kochunov, P., McGuire, S., Sherman, P., Kellner, E., ... & Fieremans, E. (2018). Evaluation of the accuracy and precision of the diffusion parameter EStImation with Gibbs and NoisE removal pipeline. NeuroImage, 183, 532-543.
14. Fischl, B. (2012). FreeSurfer. Neuroimage, 62(2), 774-781.
15. Jespersen, S. N., Olesen, J. L., Hansen, B., & Shemesh, N. (2018). Diffusion time dependence of microstructural parameters in fixed spinal cord. Neuroimage, 182, 329-342.
16. Lee, H. H., Fieremans, E., & Novikov, D. S. (2018). LEMONADE (t): Exact relation of time-dependent diffusion signal moments to neuronal microstructure. In Proc Intl Soc Mag Reson Med (Vol. 26, p. 884).
17. Nilsson, M., Lätt, J., van Westen, D., Brockstedt, S., Lasič, S., Ståhlberg, F., & Topgaard, D. (2013). Noninvasive mapping of water diffusional exchange in the human brain using filter‐exchange imaging. Magnetic resonance in medicine, 69(6), 1572-1580.
18. Yang, D. M., Huettner, J. E., Bretthorst, G. L., Neil, J. J., Garbow, J. R., & Ackerman, J. J. (2018). Intracellular water preexchange lifetime in neurons and astrocytes. Magnetic resonance in medicine, 79(3), 1616-1627.
19. Williamson, N. H., Ravin, R., Benjamini, D., Merkle, H., Falgairolle, M., O'Donovan, M. J., ... & Basser, P. J. (2019). Magnetic resonance measurements of cellular and sub-cellular membrane structures in live and fixed neural tissue. Elife, 8, e51101.
Figures