0246
Integration of blip reversal with CAIPI sampling enables simultaneous correction of slice aliasing and distortion in 3D multi-slab diffusion MRI1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom
Synopsis
3D EPI can provide optimal SNR efficiency for high-resolution diffusion MRI but is prone to aliasing of edge slices and in-plane distortion artifacts. We propose a highly-efficient method for correcting slice-aliasing and distortion for 3D multi-slab imaging without increasing scan time. Blip reversal is integrated into CAIPI sampling within a single scan. In addition, we extend the FOV along the slice direction to remove slice-aliasing artifacts. Field maps are estimated from the blip-reversed data and incorporated in the final joint reconstruction. The efficacy of the method is quantitatively and systematically validated using highly realistic simulations.
Introduction
High-resolution diffusion MRI (dMRI) allows precise assessment of tissue microstructure and structural connectivity. The primary challenge in high-resolution dMRI is the low signal-to-noise ratio (SNR). 3D multi-slab imaging is a promising technique to address this challenge by acquiring data with optimal SNR efficiency due to its compatibility with TR=1-2s1,2.Three-dimensional echo-planar imaging (3D-EPI) is one trajectory that can achieve this efficiency. 3D-EPI has two primary challenges: in-plane distortion and through-plane (slice) aliasing. The distortion artifacts are routinely corrected using a field map measured independently. However, this approach cannot capture dynamic B0 field changes due to subject motion, eddy currents, and field drift. The slice-aliasing at slab boundaries caused by the non-rectangular RF profile (Fig. 1b) can be reduced by oversampling in the slice direction or overlapping adjacent slabs, but these methods require prolonged scan time3.
We integrate Blip Up-Down Acquisition (BUDA)4 with CAIPI sampling to enable robust reconstruction and correction of distortions and slice-aliasing artifacts without increasing scan time for 3D multi-slab dMRI. The proposed method is demonstrated using simulations based on retrospectively re-sampled in vivo data.
Methods
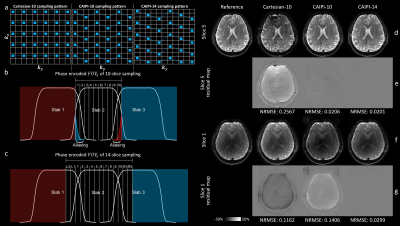
Sampling and reconstruction. Like BUDA, we acquired multi-shot EPI with interleaved blip-up/down phase encodings and estimated B0 field maps from the separately reconstructed images using FSL’s Topup5,6. We under-sampled along slice direction (kz) to obtain blip-up/down images without increasing scan time (Rz=2). To keep the motion-induced phase approximately constant across the slab, we used multiple thin slabs, resulting in similar coil sensitivity in the slice direction, which could lead to ill-conditioned reconstruction for kz under-sampling. We used CAIPI sampling to address this problem and further increased kz accelerations to extend the field-of-view (FOV) along the slice direction to remove slice-aliasing artifacts.In Fig. 1a, we compared three sampling schemes with identical acquisition time:
1) a conventional 10-slice Cartesian sampling (Cartesian-10, Ry/Rz=2/2);
2) a 10-slice CAIPI shifted acquisition (CAIPI-10, Ry/Rz=2/2) that increases the distance between aliasing voxels and reduces the g-factor penalties for reconstruction7,8(Fig.1 d,e);
3) sampling of 14 kz planes (CAIPI-14, Ry/Rz=2/2.8) to encode a wider slice FOV (Fig. 1c) to remove slice-aliasing (slices 1/10, Fig.1 f,g).
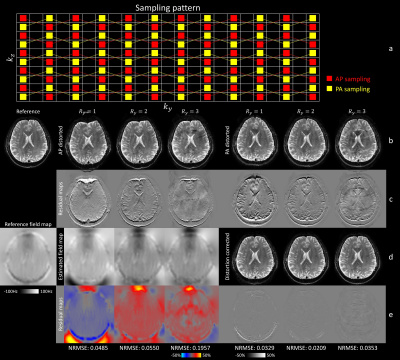
The sampling pattern for blip-reversed distortion correction was depicted in Fig. 2a. The blip-up (anterior-posterior phase encoding) and blip-down images (posterior-anterior phase encoding) were acquired using interleaved kz phase encoding with CAIPI shift. For joint de-aliasing and distortion correction, the sampling pattern extended to 14 kzplanes.
A three-stage reconstruction was used:
1) separate reconstruction of highly-accelerated blip-up/down images using SENSE9;
2) Topup5,6 field map estimation using these images;
3) the blip-up/down data were reconstructed at a lower joint acceleration using a model-based reconstruction incorporating the field map for distortion correction10 instead of being directly corrected using Topup.
Retrospective simulation. We simulated our technique using a 3D multi-slab dMRI dataset acquired using readout-segmented EPI. Acquisition parameters: FOV=220x220x111mm3, 1.5mm isotropic, TE/TR=78/2000ms, 5 readout-segments, b=1000s/mm2. “Reference” data were processed with NPEN11 for slab boundary artifacts correction and Topup and Eddy12 for distortion correction.
The multi-coil data were simulated by multiplying the reference image with 32-channel sensitivity maps. Complex Gaussian noises were added (SNR=10). Image distortions were simulated using a field map estimated from blip-reversed images and an echo spacing of 0.8ms.
Analysis. Reconstruction and field map estimation were evaluated using normalized root mean squared error (NRMSE) within a brain mask. The accuracy of the DTI metrics was quantified using mean absolute error of fractional anisotropy (FA) within the brain, and of the primary eigenvector (V1) within white matter.
Results
In Fig. 1, CAIPI sampling substantially improved the reconstruction compared to conventional Cartesian sampling. CAIPI-10 performed similarly to CAIPI-14 for central slices, but suffered from aliasing at edge slices, which were corrected in CAIPI-14.Figure 2 demonstrated our ability to robustly estimate field maps (stage 2) from highly under-sampled blip up/down images (stage 1), which were then integrated into a joint reconstruction at lower acceleration (stage 3). At stage 1, Ry=1 had the most severe distortions due to the longest effective echo spacing while Ry=3 had the most severe aliasing and the lowest SNR due to the highest under-sampling factor. Ry=2 provided the lowest NRMSE in joint (stage 3) reconstruction.
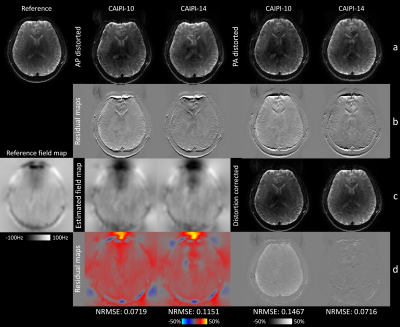
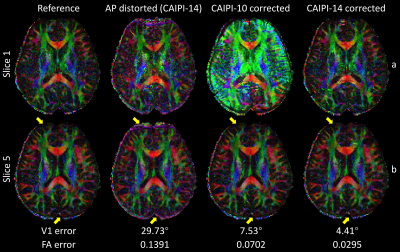
Figure 3 demonstrated joint correction of slice-aliasing and distortions. CAIPI-10 corrected the distortions but suffered from slice-aliasing at edge slices, while CAIPI-14 reduced both artifacts.
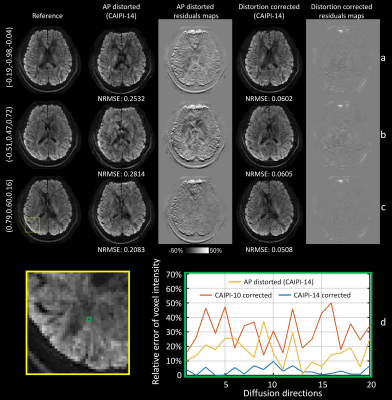
Figure 4 demonstrated results for diffusion-weighted images, where distortions were directionally dependent due to eddy currents. The corrected images were aliasing- and distortion-free, with substantially lower NRMSE and less anatomical bias than the distorted images. The signal errors of a voxel near grey-white boundary for CAIPI-14 were lower and more stable over diffusion-encoded directions than the distorted images and CAIPI-10 results, indicating removal of both slice-aliasing and eddy-current distortions.
Figure 5 showed the DTI metrics. CAIPI-14 results were similar to those from reference images. The distortions increased the variance in reconstructed images, resulting in inflated FA and inaccurate orientation estimates. CAIPI-10 results were similar to CAIPI-14 for central slices but suffered from severe slice-aliasing for edge slices.
Discussion and Conclusion
The proposed method is a promising technique to achieve high-resolution aliasing- and distortion-free 3D multi-slab imaging. It may also improve existing slab-boundary-artifacts correction methods like NPEN11 by removing slice-aliasing.Acknowledgements
W.W. is supported by the Royal Academy of Engineering (RF\201819\18\92). K.L.M. is supported by the Wellcome Trust (WT202788/Z/16/A). The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z).References
1. Frost R, Miller KL, Tijssen RH, Porter DA, Jezzard P. 3D multi‐slab diffusion‐weighted readout‐segmented EPI with real‐time cardiac‐reordered k‐space acquisition. Magnetic resonance in medicine. 2014;72(6):1565-1579.
2. Wu W, Poser BA, Douaud G, et al. High-resolution diffusion MRI at 7T using a three-dimensional multi-slab acquisition. NeuroImage. 2016;143:1-14.
3. Engström M, Skare S. Diffusion‐weighted 3D multislab echo planar imaging for high signal‐to‐noise ratio efficiency and isotropic image resolution. Magnetic resonance in medicine. 2013;70(6):1507-1514.
4. Liao C, Bilgic B, Tian Q, et al. Distortion‐free, high‐isotropic‐resolution diffusion MRI with gSlider BUDA‐EPI and multicoil dynamic B0 shimming. Magnetic Resonance in Medicine. 2021;86(2):791-803.
5. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870-888.
6. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208-S219.
7. Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi‐slice imaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2005;53(3):684-691.
8. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped‐controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g‐factor penalty. Magnetic resonance in medicine. 2012;67(5):1210-1224.
9. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 1999;42(5):952-962.
10. Zahneisen B, Aksoy M, Maclaren J, Wuerslin C, Bammer R. Extended hybrid-space SENSE for EPI: Off-resonance and eddy current corrected joint interleaved blip-up/down reconstruction. NeuroImage. 2017;153:97-108.
11. Wu W, Koopmans PJ, Frost R, Miller KL. Reducing slab boundary artifacts in three‐dimensional multislab diffusion MRI using nonlinear inversion for slab profile encoding (NPEN). Magnetic resonance in medicine. 2016;76(4):1183-1195.
12. Andersson JL, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063-1078.
Figures