0234
Top-up algorithm for single k-space from single-shot EPI1Department of Bio and Brain Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea, Republic of, 2Siemens Healthineers, Seoul, Korea, Republic of, 3Department of Radiology, Seoul National University College of Medicine, Seoul, Korea, Republic of
Synopsis
Top-up algorithm requires two EPI images with opposite gradient polarities to make one corrected image. Here, top-up algorithm with a single k-space is proposed for single-shot pseudo-centric EPI, where a k-space can be separated into two halves with opposite distortions. A field map could be estimated by applying top-up algorithm to the two halves. The estimated map compensated the distortions well while preserving inner brain structures. The proposed approach provided 1.8 times higher perfusion SNR than conventional linear EPI. The approach is promising for magnetization-prepared imaging that requires high SNR, high temporal resolution, and minimal distortion with no additional scan.
INTRODUCTION
Conventionally, B0 correction is necessary for EPI images to be free of distortions caused by B0 inhomogeneity. For the B0 correction, additional B0 maps should be calculated from GRE images with two different TEs.1 On the other hand, top-up algorithm can be applied to save additional scan time.2 This algorithm has advantages in that corrections are conducted well with no additional B0 scan. However, since a corrected image is constructed from two images with opposite blips, the temporal resolution is worsened by a factor of two. If the top-up algorithm can be applied to a single-shot EPI, it would eliminate requirement of additional scan, while maintaining the temporal resolution. This might become possible if two opposite distortions co-exist in a single k-space data. Recently, EPI with novel trajectory, single-shot pseudo-centric EPI (1sh-CenEPI)3, is introduced, where two halves of a k-space can be separated into two opposite distortions and then converted into the image domain. In this study, application of the top-up algorithm to single k-space from 1sh-CenEPI is introduced for magnetization-prepared imaging to maintain high SNR, high temporal resolution, and minimal distortion with no additional scan.METHODS
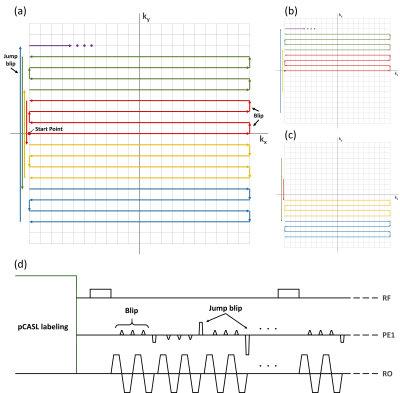
(Background) 1sh-CenEPI3 has a 2D trajectory, where center lines of the k-space are acquired first. Then the other lines are filled with series of alternate blips and jump blips in every four lines (Figure 1.(a)) In addition, it has the unique characteristics in that the k-space can be divided into two halves depending on blip directions, which determine the distortion directions as well. (Figure 1.(b),(c)) This trajectory was applied to 2D EPI in the previous study. In this study it is extended to each partition k-space for 3D EPI. The sequence diagram for 3D 1sh-CenEPI is represented in Figure 1.(d).(Data acquisition) To investigate the effectiveness of the proposed approach in magnetization-prepared imaging, pseudo-continuous arterial spin labeling (pCASL) was conducted with the proposed 3D 1sh-CenEPI with single labeling/control period.4 The parameters were the same as those of the previous study.4 As comparison reference, pCASL with 3D EPI with linear trajectory was also scanned.
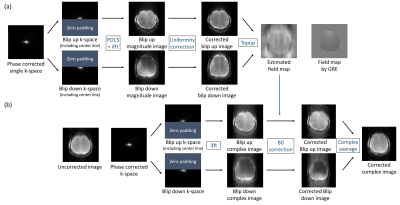
(Post processing) To correct the distortions caused by time gap for jump blips, the whole echo phase correction3 was applied. For the next step to estimate the field map, two images with opposite distortions were extracted from the single k-space following these steps (Figure 2.(a)): 1) the single k-space was separated into two k-spaces depending on the blip directions. In this step, the center k-space line was included in both blip up and down k-spaces to maintain reasonable signal intensities. 2) POCS5 and uniformity correction6 were applied to compensate for aliasing and signal nonuniformity7 caused by partial k-space. These corrections and the duplicate inclusions of the center line were applied only for the field map estimation, but not for the composition of the main images. Then, the B0 map was estimated by top-up algorithm with these two processed images. Finally, with the estimated map, the final corrected image was obtained by the B0 correction that was applied to the two halves of the k-space separately, which were then summed in the complex images, as suggested for the 1sh-CenEPI.3 (Figure 2.(b)) These processes were conducted using MATLAB and FSL8.
The perfusion-weighted image was calculated by subtracting label images from control images and by averaging them.9 For the quantitative comparison between linear and 1sh-CenEPI, SNR was calculated by dividing average gray matter signal by standard deviation of background in the perfusion weighted image.10
RESULTS
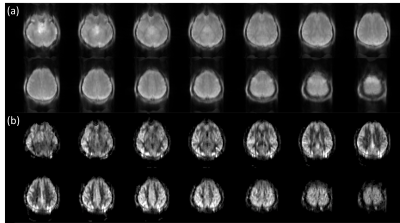
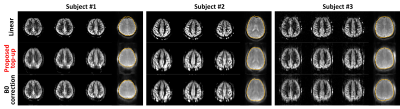
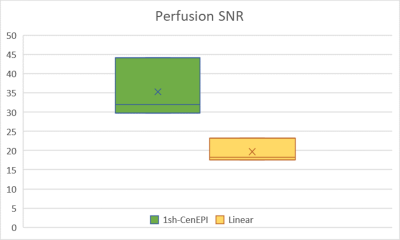
The representative corrected baseline images and perfusion-weighted images are shown in Figure 3. In addition, 1sh-CenEPI with the proposed correction was compared with the linear EPI without correction (Figure 4), under the same condition of no additional B0 scan. The superiority of the proposed method in distortion correction was well observed as shown by the GRE-based ground-true boundary (orange in Figure 4), which was comparable to the correction with the additional B0 scan (Figure 4). While the proposed top-up algorithm might cause slight blurring as shown in the perfusion-weighted images, overall inner structures were preserved well. SNR of the perfusion-weighted images for the proposed 1sh-CenEPI was much higher (1.8 times higher) than that of the linear EPI. (Figure 5)DISCUSSION & CONCLUSION
In this study top-up algorithm is proposed for single k-space from 1sh-CenEPI. This method has great advantage in that distortion correction can be performed with its own single k-space information. It provides high temporal resolution and saves the scan time due to no necessity of additional scan. However, further improvement is required as to some blurring effects, which were presumably related to the B0 map estimation (Figure 2.(a)).Furthermore, since 1sh-CenEPI acquires the center part of the k-space first, it can enhance SNR for magnetization-prepared imaging3 as demonstrated in Figure 5. Based on the high SNR difference, it is expected that the SNR advantage would be maintained even after the blurring effects are resolved.
In conclusion, the proposed top-up algorithm for 1sh-CenEPI has great potential to be applicable as a readout for magnetization prepared imaging that requires high SNR, high temporal resolution, and minimal distortion with no additional scan such as functional perfusion imaging.
Acknowledgements
No acknowledgement found.References
1. Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magnetic resonance in medicine. 1995;34(1):65-73.
2. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208-S219.
3. Lee HS, Hwang SH, Park J, Park SH. Single‐shot pseudo‐centric EPI for magnetization‐prepared imaging. Magnetic Resonance in Medicine. 2021.
4. Hwang S-H, Han S, Kim S-G, Park J, Park S-H. Whole-brain Perfusion Mapping at 7T by SAR-efficient Non-segmented 3D EPI-pCASL. 2021 ISMRM & SMRTANNUAL MEETING & EXHIBITION; 2021.
5. McGibney G, Smith M, Nichols S, Crawley A. Quantitative evaluation of several partial Fourier reconstruction algorithms used in MRI. Magnetic resonance in medicine. 1993;30(1):51-59.
6. Cohen MS, DuBois RM, Zeineh MM. Rapid and effective correction of RF inhomogeneity for high field magnetic resonance imaging. Human brain mapping. 2000;10(4):204-211.
7. Chen Nk, Oshio K, Panych LP. Improved image reconstruction for partial fourier gradient‐echo echo‐planar imaging (EPI). Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2008;59(4):916-924.
8. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782-790.
9. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic resonance in medicine. 2015;73(1):102-116.
10. Xu G, Rowley HA, Wu G, et al. Reliability and precision of pseudo‐continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O‐water PET in elderly subjects at risk for Alzheimer's disease. NMR in Biomedicine. 2010;23(3):286-293.
Figures