0232
Distortion- and ghosting-free high b-value ex vivo human brain diffusion MRI achieved with spatiotemporal magnetic field monitoring1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States, 2Skope Magnetic Resonance Technologies AG, Zurich, Switzerland, 3Skope Magnetic Resonance Inc, Lake Mills, WI, United States, 4Institute of Medical Physics and Radiation Protection, Giessen, Germany, 5Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, United States, 6Q Bio Inc,, San Carlos, MA, United States
Synopsis
Whole brain ex vivo diffusion MRI needs high b-value encoding to compensate for the reduced diffusivity, requiring human brain scanners with ultra-strong diffusion gradients. At that regime, eddy currents become too severe, to the point image distortions and ghosting artifacts cannot be enough mitigated with conventional image reconstruction and post-processing approaches. On a 3T Connectom scanner , we demonstrate the importance of spatiotemporal field monitoring to measure and model eddy currents. When Fourier reconstruction is informed with the unwanted spatiotemporal evolution of the phase, distortion- and ghosting-free high-resolution DW images can be achieved.
Introduction
Ex vivo diffusion MRI (dMRI) is a key tool for probing structural connectivity and microstructure in the human brain at the mesoscopic scale as it permits lengthy acquisitions with unparalleled spatial resolution with high signal-to-noise ratio (SNR)1. The shortened T2 relaxation times and reduced diffusivity of ex vivo tissue2 require the use of stronger diffusion-encoding gradients to achieve diffusion-weighted contrast comparable to in vivo scans 3,4,5,6. In the regime of very high gradient strengths, eddy currents become more pronounced7 and can lead to severe image distortions and ghosting artifacts, confounding the estimation of diffusion MRI metrics in fine-scale structures8In this work, we show the benefits of using spatiotemporal magnetic field monitoring to measure and model the severe eddy currents caused by strong diffusion encoding on a 3T Connectom scanner. We demonstrate the efficacy of this approach in reducing artifacts such as eddy current-induced distortions and ghosting, where conventional image reconstruction and post-processing approaches are insufficient.
Methods
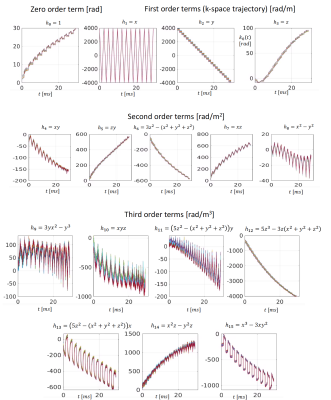
We measured the field evolution of the magnetic field for an ex vivo diffusion MRI scan with a dynamic field camera (Skope, Zurich) 9,10,11,12 which consists of 16 NMR probes. A combined model composed of the real-valued solid spherical harmonic expansion up to third order and a concomitant field term is fitted to the phase data measured in the NMR probes, rendering the following smooth approximation for the phase evolution $$$\phi(\textbf{r},t)$$$:$$\phi(\textbf{r},t) \approx \gamma B_{0}t + {\sum}_{l=0}^{15}k_l(t)h_l(\textbf{r}) + {\sum}_{l=0}^{3}k_{{con}_l}(t)h_{{con}_l}(\textbf{r})$$
where $$$h_l(\textbf{r})$$$ are the real-valued solid spherical harmonics up to third order, see Fig1, (note that the first three are a constant term and the actual k-space trajectories along x, y, and z respectively) and $$$h_{{con}_l}(\textbf{r})$$$ are the concomitant field components ( with components $$$k_{{con}_l}(t)$$$)
The forward multi-channel reconstruction model incorporating higher-order field terms can be written as
$$ \textbf{y}_p = \textbf{E}\textbf{C}_p\textbf{x} \quad p=1,...,P $$
where $$$\textbf{y}_p = {[\textbf{y}_p(t_1), \textbf{y}_p(t_2),..., \textbf{y}_p(t_n),...,\textbf{y}_p(t_N)]}^T$$$ is the k-space data of the p-th channel acquired at time points $$$t_n$$$, $$$\textbf{E} = \{\textbf{E}_{m,n}\}$$$ with $$$\textbf{E}_{m,n} = e^{i{\gamma}\phi(\textbf{r}_m,t_n)}$$$, $$$\textbf{C}_p$$$ is the diagonal matrix representing coil sensitivity, and $$$\textbf{x}$$$ the image $$$S(\textbf{r})$$$ discretized at points $$$\textbf{r}_m$$$ ($$$m=1,...,M$$$) which can be estimated by solving the following Tikhonov regularization problem
$$\min_{\textbf{x}} \sum_{p=1}^P {\vert \vert \textbf{y}_p - \textbf{E}\textbf{C}_p\textbf{x}\vert\vert}_2 + \lambda{\vert \vert \textbf{x} \vert \vert}_2$$
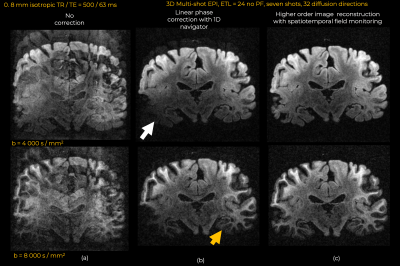
Coil sensitivities are estimated from a gradient echo acquisition with the ESPIRiT algorithm13. We compare this phase-informed advanced higher-order reconstruction approach with the conventional reconstruction pipeline, which consists of: standard linear phase correction (LPC) based on 1D navigators 14 to mitigate ghosting; coil combination with SENSE; and image-space distortion correction with the FSL tool “eddy”15.
Data:
All data were acquired on the 3T Connectom MRI scanner (MAGNETOM Connectom, Siemens) with maximum gradient strength of 300 mT/m. A custom-made 48-channel coil for ex vivo whole brain imaging was used for signal reception16.Diffusion MRI data were acquired from a coronal slab within the center of an ex-vivo fixed whole human brain specimen. Images were acquired at 0.8 mm isotropic resolution with a Stejskal-Tanner, 3D multi-shot EPI sequence, with FOV = 392x133x8 mm, matrix size = 492x168x10, TR/TE = 500/63 ms, echo train length=24, echo spacing 1.22 ms, seven shots, no partial Fourier. Multi-shell dMRI data were acquired with b-values of b=4000, 8000, 12000 s/mm2 (gradient strengths of 140, 214, and 285mT/m) with 32 non-collinear diffusion directions and two b0-images per shell. An additional 0.8 mm isotropic dataset was acquired with b=50,000 s/mm2 ( 282mT/m), TE = 79 ms, and all other acquisition parameters as above.
Results
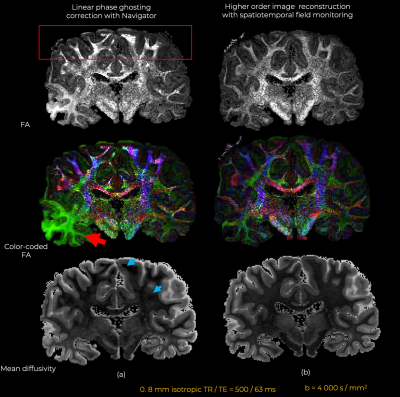
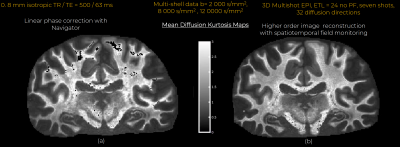
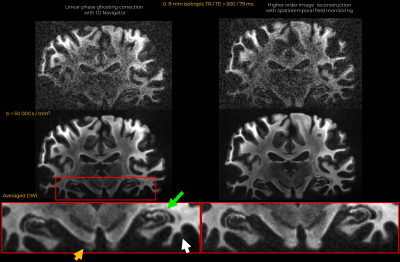
Eddy currents at high gradient strengths create strong higher-order encoding terms (Fig.1b) that manifest as severe ghosting in the reconstructed images (Fig.2). With conventional reconstruction, residual ghosting limited the ability to resolve important anatomical areas such as the temporal lobes and hippocampus. In the images reconstructed with dynamic field monitoring, these areas were free of ghosting artifacts. Residual artifacts in the DWIs obtained with conventional reconstruction led to noticeable artifacts in the DTI (Fig.3) and DKI (Fig.4) maps. These artifacts were removed with model-based distortion correction enabled by dynamic field monitoring. The average DWIs shown in Fig.5 demonstrate the blurring resulting from spatial interpolation using ‘eddy’, which leads to substantial loss of resolution, e.g., in the hippocampus. When both ghosting and eddy-current effects were accounted for in the forward model, there was no blurring in the average DWI, even at b = 50,000 s/mm2.Discussion and Conclusions
In the ultra-high-gradient strength regime, strong eddy currents lead to severe image distortion and ghosting that cannot be eliminated by conventional, image-space distortion correction techniques like ‘eddy’. As a result, model-based reconstruction techniques are critical for enabling high-fidelity, high-resolution whole brain ex-vivo diffusion MRI at ultra-high b-value. In this work, we have shown that model-based reconstruction with a priori dynamic monitoring of the magnetic field is more effective at reducing ghosting artifacts and image distortions than conventional methods. Removing these artifacts improves the quality of downstream analyses. Our results show the importance of field monitoring for mesoscopic diffusion MRI using ultra-high-gradient strengths. Though the current work focused on post-mortem imaging, we expect in-vivo diffusion MRI with high gradient strength systems such as the Connectom scanner to also benefit from the phase-informed image reconstruction approach shown here.Acknowledgements
This work was supported by the NIH grants ( P41-EB030006, U01-EB026996, R01-EB021265, K23-NS096056, R01 EB028797, R03 EB031175, U01 EB025162, P41 EB030006, the Office of the Director of the NIH under award DP5 OD031854 ), and the NVidia Corporation for computing support.References
1Roebroeck A, Miller KL, Aggarwal M. Ex vivo diffusion MRI of the human brain: Technical challenges and recent advances. NMR Biomed. 2019 Apr;32(4):e3941. doi: 10.1002/nbm.3941. Epub 2018 Jun 4. PMID: 29863793; PMCID: PMC6492287.
2Shepherd, T., Thelwall, P., Stanisz, G., & Blackband, S. (2009). Aldehyde fixative solutions alter the water relaxation and diffusion properties of nervous tissue. Magn. Reson. Med., 62(1), 26-34.
3Huang SY, Witzel T, Keil B, Scholz A, Davids M, Dietz P, Rummert E, Ramb R, Kirsch JE, Yendiki A, Fan Q, Tian Q, Ramos-Llordén G, Lee HH, Nummenmaa A, Bilgic B, Setsompop K, Wang F, Avram AV, Komlosh M, Benjamini D, Magdoom KN, Pathak S, Schneider W, Novikov DS, Fieremans E, Tounekti S, Mekkaoui C, Augustinack J, Berger D, Shapson-Coe A, Lichtman J, Basser PJ, Wald LL, Rosen BR. Connectome 2.0: Developing the next-generation ultra-high gradient strength human MRI scanner for bridging studies of the micro-, meso- and macro-connectome. Neuroimage. 2021 Nov;243:118530. doi: 10.1016/j.neuroimage.2021.118530. Epub 2021 Aug 28. PMID: 34464739.
4McNab, J. A., Jbabdi, S., Deoni, S. C., Douaud, G., Behrens, T. E., & Miller, K. L. (2009). High resolution diffusion-weighted imaging in fixed human brain using diffusion-weighted steady state free precession. Neuroimage, 46(3), 775-785.
5Miller, K. L., McNab, J., Jbabdi, S., & Douaud, G. (2012). Diffusion tractography of post-mortem human brains: optimization and comparison of spin echo and steady-state free precession techniques. Neuroimage, 59(3), 2284-2297.
6Miller, K. L., Stagg, C. J., Douaud, G., Jbabdi, S., Smith, S. M., Behrens, T. E., . . . McNab, J. (2011). Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage, 57(1), 167-181.
7Setsompop, K., Kimmlingen, R., Eberlein, E., Witzel, T., Cohen-Adad, J., McNab, J., . . . Wald, L. (2013). Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. Neuroimage, 80, 220-233.
8Ramos-Llordén, G., Lobos, R., Kim, T. H., Tian, Q., Witzel, T., Lee, H. H., ... & Huang, S. Y. (2021). High-fidelity, high-spatial-resolution diffusion MRI of the ex-vivo whole human brain on the 3T Connectom scanner using structured low-rank EPI ghost correction. bioRxiv. https://doi.org/10.1101/2021.08.01.454635
9Dietrich BE, Brunner DO, Wilm BJ, Barmet C, Gross S, Kasper L, Haeberlin M, Schmid T, Vannesjo SJ, Pruessmann KP. A field camera for MR sequence monitoring and system analysis. Magn Reson Med. 2016 Apr;75(4):1831-40. doi: 10.1002/mrm.25770. Epub 2015 May 14. PMID: 25975352.
10Barmet C, De Zanche N, Pruessmann KP. Spatiotemporal magnetic field monitoring for MR. Magn Reson Med. 2008 Jul;60(1):187-97. doi: 10.1002/mrm.21603. PMID: 18581361.
11Wilm BJ, Barmet C, Pavan M, Pruessmann KP. Higher order reconstruction for MRI in the presence of spatiotemporal field perturbations. Magn Reson Med. 2011 Jun;65(6):1690-701. doi: 10.1002/mrm.22767. Epub 2011 Apr 22. PMID: 21520269.
12Barmet C, Wilm BJ, Pavan M, Pruessmann K. A third-order field camera with microsecond resolution for MR system diagnostics. Proceedings ISMRM, 17th Scientific Meeting and Exhibition, Honolulu; 2009.
13Uecker M, Lai P, Murphy MJ, et al. ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med. 2014;71(3):990-1001. doi:10.1002/mrm.24751
14Buonocore, M. H., & Gao, L. (1997). Ghost artifact reduction for echo planar imaging using image phase correction. Magnetic resonance in medicine, 38(1), 89-100.
15Jesper L. R. Andersson and Stamatios N. Sotiropoulos. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125:1063-1078, 2016.
16Scholz A, Etzel R, May MW, Mahmutovic M, Tian Q, Ramos-Llordén G, Maffei C, Bilgiç B, Witzel T, Stockmann JP, Mekkaoui C, Wald LL, Huang SY, Yendiki A, Keil B. A 48-channel receive array coil for mesoscopic diffusion-weighted MRI of ex vivo human brain on the 3 T connectome scanner. Neuroimage. 2021 Sep;238:118256. doi: 10.1016/j.neuroimage.2021.118256. Epub 2021 Jun 9. PMID: 34118399; PMCID: PMC8439104.
Figures