0230
Real-time 3D MRI for Low-Latency Volumetric Motion Tracking on a 1.5T MR-Linac System1Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 3Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
Real-time 3D MRI for low-latency volumetric motion tracking was successfully implemented on a 1.5T MR-Linac system using the MRSIGMA framework. A first scan was performed for offline learning to obtain a training dictionary of ten 3D motion states. A second scan with the same sequence parameters was performed to generate the motion signature in real-time for online matching and was also used as a reference for retrospective self-validation. The feasibility of the technique was demonstrated on a healthy volunteer and a patient with pancreatic cancer which presented high quantitative concordance between contours of real-time MRSIGMA matching and the reference.
INTRODUCTION
The MR-Linac system offers opportunities for real-time adaptive treatment for tumors affected by continuous motion, such as those located in the upper abdomen which are affected by respiratory and digestive motion.1,2 Real-time adaptive treatment holds the promise to focus the radiation beam to the tumor and spare nearby organs at risk which can enable definitive doses to be given. To achieve this goal, real-time 3D MRI with sufficient volumetric coverage to include the tumor and organs at risk is required to track motion and shape the radiation beam accordingly. However, current real-time MRI technology on the MR-Linac is limited to 2D imaging, which limits the performance of motion tracking. This work presents the implementation of the MR signature matching (MRSIGMA)3,4 technique on a 1.5T MR-Linac system to enable low-latency 3D MRI to track motion of pancreatic tumors and organs at risk.METHODS
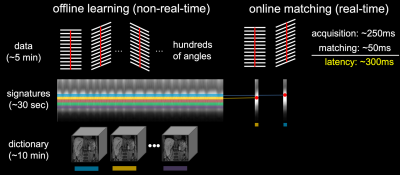
Concept of MRSIGMA: MRSIGMA shifts all the acquisition and reconstruction burden to an offline learning step (Figure 1), where continuously acquired stack-of-stars data (~5min) are reconstructed into 3D motion states using XD-GRASP.5 The motion range along the z dimension in each motion state is the motion signature. After offline learning, MRSIGMA moves to a real-time online signature matching step, where the same acquisition is employed, but for each angle (~250ms), a motion signature is computed and matched to one of the pre-computed 3D motion states (~50ms), enabling low-latency 3D MRI.MRSIGMA Data Acquisition: MRSIGMA data was acquired from a healthy volunteer (female, 31 years old) and a patient with pancreatic cancer (male, 59 years old) on a 1.5T Unity MR-Linac System (Elekta AB, Stockholm, Sweden). A free-breathing T1-weighted 3DVANE sequence was modified for 3D radial golden-angle stack-of-stars acquisitions. A training dataset was first acquired for offline learning and a second scan with the same sequence parameters was performed to generate motion signature for online matching and was also used as a reference for retrospective self-validation. Data acquisition parameters are as follows: TR/TE = 5.0/2.1 ms, flip angle = 12°, FOV = 370×370×208 mm3, voxel size = 1.5×1.5×4.0 mm3, number of radial spokes = 905, bandwidth = 720 Hz/pixel, total scan time = 5:50 min. The latency of acquiring a motion signature (one angle) is about 275 ms.
MRSIGMA Image Reconstruction: K-space data in raw data format (.data/.list files) was transferred to a high-performance computer and MRSIGMA reconstruction of 4D images (10 motion states of 3D volume) was performed in Matlab (MathWorks, Natick, MA) using in-house algorithms, as described previously.3,4
Image Contouring: Clinical contours of the patient were transferred to the first phase (expiration) of the two 4D image sets after a fusion of a clinical T1-weighted scan and the two MRSIGMA scans. The contours were then automatically propagated from the first phase to all remaining phases using a deformable propagation algorithm in the MIM software (MIM Software Inc, Cleveland, OH) and were visually inspected thereafter for minor manual adjustment.
Motion Tracking Performance Evaluation: Gross tumor volume (GTV) of the pancreas, the duodenum and stomach (Duo-stomach) as the high-risk organs, and three other nearby organs at risk (small and large bowel, and liver) were selected for evaluation. Dice coefficient was calculated for all contours of the same organ between the online matching and the reference.
RESULTS
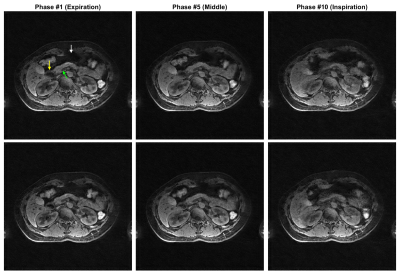
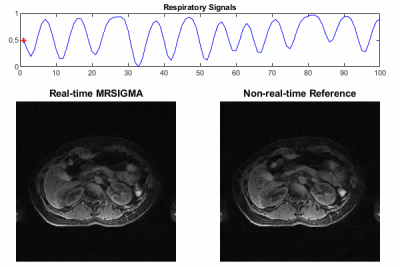
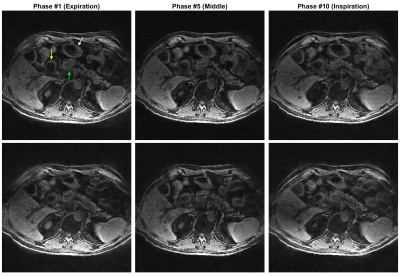
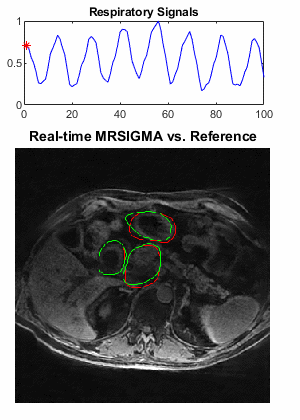
Selected motion states (expiration, middle and expiration phases) of the offline training dictionary and non-real-time reference are shown in Figure 2 and Figure 4 for the healthy volunteer and patient, where the pancreas, duodenum, and stomach are indicated by arrows in green, yellow, and white, respectively. Motion of these organs from expiration to inspiration can be readily seen for both scans. Figure 3 illustrates a good agreement of organ motion between online matching and the reference as synchronized by the motion signature. Figure 5 shows the change of the contours (only GTV and Duo-stomach are shown) for online signature matching in red, which is in good agreement with the contours of the reference in green. The Dice coefficients of the contours are: 0.912±0.016 (GTV), 0.863±0.032 (Duo-stomach), 0.876±0.061 (small bowel), 0.777±0.036 (large bowel), and 0.963±0.007 (liver), further confirming that online signature matching agrees well with the reference.DISCUSSION
The preliminary work demonstrated the feasibility of real-time 3D MRI for low-latency volumetric motion tracking on a 1.5T MR-Linac system using the MRSIGMA framework. This opens the door for adaptive planning in radiation therapy with smaller treatment margins and more focused dose delivery. There are a few ways to further reduce the latency of acquiring a motion signature, for example, using a lower acquisition resolution, reducing the number of slices to only cover the lung-liver interface, or using a pencil beam liver-lung-navigator. Further work is needed to implement a streamlined workflow for fast raw-data transfer and deep-learning-based image reconstruction to achieve total latency below 300 ms for real-time motion tracking in radiation therapy.CONCLUSION
Real-time 3D MRI for low-latency volumetric motion tracking was successfully implemented on a 1.5T MR-Linac system using an online matching of the motion signature to the offline training dictionary. The feasibility was demonstrated on a healthy volunteer and a patient with pancreatic cancer with good motion tracking performance.Acknowledgements
The work was supported by NIH Grant R01CA255661.References
1. Mutic S, Dempsey JF. The ViewRay system: Magnetic Resonance‐guided and controlled radiotherapy. Semin Radiat Oncol. 2014; 24(3):196-199.
2. Raaymakers BW, Lagendijk JJW, Overweg J, et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol. 2009. 54(12):N229-N237.
3. Feng L, Tyagi N, Otazo R. MRSIGMA: Magnetic Resonance SIGnature MAtching for real-time volumetric imaging. Magn Reson Med. 2020; 84(3):1280-1292.
4. Kim N, Tringale KR, Crane C, et al. MR SIGnature MAtching (MRSIGMA) with retrospective self-evaluation for real-time volumetric motion imaging. Phys Med Biol. 2021 Oct 26;66(21).
5. Feng L, Axel L, Chandarana H, et al. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med 2016;75(2):775-788.
Figures