0226
Diffusion-weighted MRI with deep learning for visualizing treatment results of MR-guided HIFU ablation of uterine fibroids.1Radiology, Isala Zwolle, Zwolle, Netherlands, 2Image Sciences Institute, Imaging & Oncology Division, University Medical Center Utrecht, Utrecht, Netherlands, 3Faculty of Medicine and University Hospital of Cologne, Institute of Diagnostic and Interventional Radiology, University of Cologne, Cologne, Germany, 4High Tech Campus, Philips Research Eindhoven, Eindhoven, Netherlands
Synopsis
The non-perfused volume (NPV) cannot be assessed repeatedly with contrast-enhanced imaging during MR-HIFU ablations of uterine fibroids, due to contrast agent dose constraints and safety concerns. In this study, synthetic contrast-enhanced (CE)-T1w scans were generated from diffusion weighted imaging (DWI) using deep learning-based image-to-image translation. A significant linear association was found between the NPV-ratios based on synthetic and paired reference CE-T1w scans (r=0.80, p<0.001). Radiologists agreed in 83% on treatment success based on synthetic and reference CE-T1w scans. This indicates that translation of DWI into synthetic CE-T1w scans has potential as method for gadolinium-free imaging of the NPV.
Introduction
The assessment of technical therapy success of magnetic resonance-guided high intensity focused ultrasound (MR-HIFU) ablations of uterine fibroids (UFs) is generally based on the non-perfused volume (NPV)-ratio, i.e. the NPV divided by the entire UF volume. A high NPV-ratio indicates a successful treatment1–3, and is standardly measured using gadolinium (Gd)-based contrast-enhanced (CE)-T1w imaging. However, no method is currently available to determine the NPV repeatedly during MR-HIFU ablations of UFs, as repeated acquisition of CE-T1w scans is inhibited by contrast agent dose constraints and safety concerns4,5. It has been shown that perfusion information can alternatively be retrieved from diffusion weighted imaging (DWI) using deterministic approaches including intravoxel incoherent motion analysis (IVIM), but remains challenging6,7,16–20,8–15. An alternative approach to subtract UF perfusion from DWI could possibly be deep learning-based translation into CE-MRI contrast. Therefore, we trained and evaluated a deep learning-based conditional generative adversarial (cGAN) network to perform mapping of DWI to CE-MRI scans, composing synthetic CE-T1w scans, as strategy for Gd-free imaging of the NPV after MR-HIFU ablations of UF.Methods
In this retrospective study, data from fifty-six women who underwent an MR-HIFU treatment of symptomatic UFs was used. Paired DWI and CE-T1w scans were acquired directly after the HIFU procedure at a 1.5T MR-system (Achieva, Philips Healthcare, Best, The Netherlands). The DWI protocol existed of fat-suppressed multi-slice single-shot spin echo-echo planar sequence (TR/TE = 1352ms/65ms, acquired voxel size: 2.50x4.36x6.00 mm3, reconstructed voxel size: 0.89x0.90x6.00 mm3), at different strengths of diffusion weighting corresponding to b-values of 0, 50, 100, 200, 400, 600 and 800 s/mm2. Paired CE-T1w scans were acquired using a fat-suppressed 3-D spoiled gradient-echo sequence (TR/TE/FA = 5.4ms/2.6ms/45°, acquired voxel size: 1.49x1.58x3.00 mm3, reconstructed voxel size: 0.49x0.49x1.50 mm3). In both sequences, fat suppression with an adiabatic spectrally selective inversion recovery prepulse was used. A Gd-based contrast agent (DOTAREM, 0.2mL / kg, Gadoterate Meglumine, 0.1 mmol/kg, Geurbet, Aulnay-sous-Bois, France) was injected 3 minutes prior to the CE-T1w scans.The cGAN was trained and validated on 36/56 patients (1559/2600 slices) to translate axial patches (128x128 pixels) of DWI scans to axial patches of CE-T1w scans, resulting in synthetic CE-T1w patches. Final synthetic CE-T1w scans were constituted by stitching synthetic CE-T1w patches. The cGAN concerned an image-to-image translator, based on the pix2pix implementation by Isola et al. (2017)21.
Final evaluation of synthetic CE-T1w scans was performed on 22/56 patients (1041/2600 slices). Quantitative analysis included calculation of the voxelwise error between synthetic and reference CE-T1w scans. Dice coefficient was calculated between NPVs delineated on synthetic and reference CE-T1w scans. As qualitative evaluation, four radiologists with five years’ experience in performing MR-HIFU treatments independently assessed whether the MR-HIFU treatments were technically successful, based on synthetic and reference CE-T1w scans. In addition, they estimated the NPV-ratio on both scans.
Results
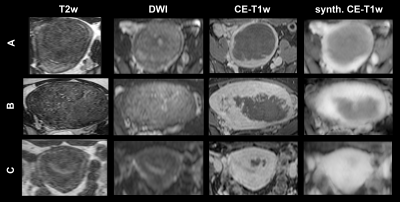
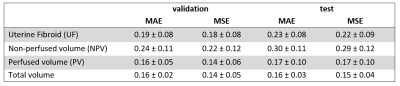
Three representative examples of uterine fibroids from the test dataset are presented in Figure 1, including an anatomical T2w, DWI, synthetic CE-T1w and reference CE-T1w slice.On average, total volume MAE and MSE between synthetic and reference CE-T1w scans in the test set were 0.16 (± 0.03) and 0.15 (± 0.04), respectively (Table 1).
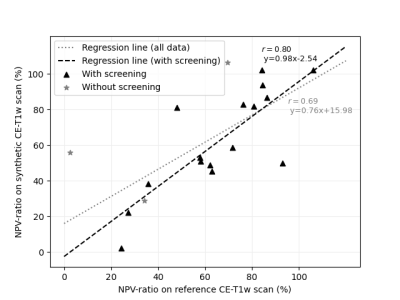
Dice coefficient between the NPVs delineated on the synthetic and reference CE-T1w scans was on average 0.71 (± 0.22). The NPV-ratios were not statistically different (p = 0.79). There was a significant linear relationship between the NPV-ratios delineated on the reference and synthetic CE-T1w scans (p<0.001) (Figure 2).
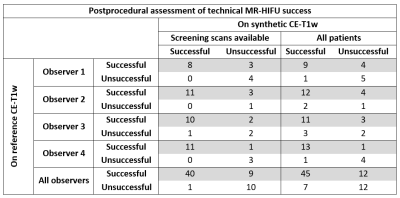
The absolute intra-observer agreement on assessment of technical treatment success between synthetic and reference CE-T1w scans when screening synthetic CE-T1w scans were available was 83% (n = 15) (Table 2). For the entire test set, including cases without screening synthetic CE-T1w scans, the absolute agreement was 75% (n = 19). The mean estimated NPV-ratio on the synthetic CE-T1w scan was 64.3% (± 23.1%) and 72.2% (± 22.9%) on the reference CE-T1w scans. The mean ground-truth NPV-ratio was 69.1% (± 32.6%). The estimated NPV-ratio on the synthetic CE-T1w scans was not significantly different from the estimated NPV-ratio on reference CE-T1w scans (p = 0.27) or the ground-truth NPV-ratios (p = 0.60)
Discussion
The findings from this study suggest that the proposed method in this study has potential to play an essential role in allowing repeated visualization of the NPV during MR-HIFU procedures. The assessment of radiologists on technical treatment success based on synthetic and reference CE-T1w scans showed good agreement. Looking forward, in order to validate this method for its intended use of intraprocedural monitoring of treatment progression, a prospective intraprocedural investigation should be performed, including smaller NPVs observed in early phases of the treatment procedures. Additionally, external validation of this approach is necessary for adoption in other MR-HIFU centers. For technical improvements of synthetic CE-T1w scans, efforts could focus on optimizing DWI sequences to increase spatial resolution, e.g. by using DWI scans with smaller voxels as input data for the DL network.Conclusion
Deep learning-based synthetic CE-T1w scans derived from DWI allow postprocedural Gd-free visualization of the predicted NPV. Synthetic CE-T1w scans can potentially be used for repeated per-procedural assessment of the treatment effect during MR-HIFU therapy for uterine fibroids, as DWI can be repeatedly acquired during the procedure.Acknowledgements
NAReferences
1. Verpalen, I. M. et al. The Focused Ultrasound Myoma Outcome Study (FUMOS); a retrospective cohort study on long-term outcomes of MR-HIFU therapy. Eur. Radiol. 30, 2473–2482 (2020).
2. Verpalen, I. M. et al. Magnetic resonance-high intensity focused ultrasound (MR-HIFU) therapy of symptomatic uterine fibroids with unrestrictive treatment protocols: A systematic review and meta-analysis. Eur. J. Radiol. 120, 108700 (2019).
3. Keserci, B. & Duc, N. M. The role of T1 perfusion-based classification in magnetic resonance-guided high-intensity focused ultrasound ablation of uterine fibroids. Eur. Radiol. 27, 5299–5308 (2017).
4. Hijnen, N. M. et al. The magnetic susceptibility effect of gadolinium-based contrast agents on PRFS-based MR thermometry during thermal interventions. J. Ther. Ultrasound 1, 1–9 (2013).
5. Hijnen, N. M., Elevelt, A. & Grüll, H. Stability and trapping of magnetic resonance imaging contrast agents during high-intensity focused ultrasound ablation therapy. Invest. Radiol. 48, 517–524 (2013).
6. Hectors, S. J. C. G. et al. Multiparametric MRI analysis for the evaluation of MR-guided high intensity focused ultrasound tumor treatment. NMR Biomed. 28, 1125–1140 (2015).
7. Zimmerman, B. E. et al. Learning Multiparametric Biomarkers for Assessing MR-Guided Focused Ultrasound Treatment of Malignant Tumors. IEEE Trans. Biomed. Eng. 68, 1737–1747 (2021).
8. Jacobs, M. A., Herskovits, E. H. & Kim, H. S. Uterine fibroids: Diffusion-weighted MR imaging for monitoring therapy with focused ultrasound surgery - Preliminary study. Radiology 236, 196–203 (2005).
9. Giles, S. L. et al. Value of diffusion-weighted imaging for monitoring tissue change during magnetic resonance-guided high-intensity focused ultrasound therapy in bone applications: an ex-vivo study. Eur. Radiol. Exp. 2, 10 (2018).
10. Chetan, M. R. et al. Role of diffusion-weighted imaging in monitoring treatment response following high-intensity focused ultrasound ablation of recurrent sacral chordoma. Radiology case reports vol. 14 1197–1201 (2019).
11. Walker, M. R. et al. Acute MR-Guided High-Intensity Focused Ultrasound Lesion Assessment Using Diffusion-Weighted Imaging and Histological Analysis. Front. Neurol. 10, 1069 (2019).
12. Verpalen, I., Boomsma, M., Edens, M. & Heijman, E. The evaluation of the Non-Perfused Volume after MR-HIFU treatment of uterine fibroids using quantitative T2-mapping and diffusion weighted imaging. in 19th International Symposium of ISTU and 5th European Symposium of EUFUS 143 (2019).
13. Ikink, M. E. et al. Diffusion-weighted magnetic resonance imaging using different b-value combinations for the evaluation of treatment results after volumetric MR-guided high-intensity focused ultrasound ablation of uterine fibroids. Eur. Radiol. 24, 2118–2127 (2014).
14. Ikink, M. E. et al. IntraVoxel Incoherent Motion MRI for the characterization of uterine fibroids before MR-guided high-intensity focused ultrasound ablation. in Proceedings of the Joint Annual Meeting International Society for Magnetic Resonance In Medicine - European Society for Magnetic Resonance in Medicine and Biology 3693 (2014).
15. Verpalen, I. M. Diffusion-Weighted Imaging to monitor treatment progression of Magnetic Resonance guided Focused Ultrasound Fibroid Ablation. Thesis: Improving treatment efficacy of MR-HIFU fibroid ablation 131-148 (Chapter 6) (2021).
16. Iima, M. & Le Bihan, D. Clinical intravoxel incoherent motion and diffusion MR imaging: Past, present, and future. Radiology vol. 278 13–32 (2016).
17. Bihan, D. Le & Turner, R. The capillary network: a link between ivim and classical perfusion. Magn. Reson. Med. 27, 171–178 (1992).
18. Dijkstra, H., Oudkerk, M., Kappert, P. & Sijens, P. E. Assessment of the link between quantitative biexponential diffusion-weighted imaging and contrast-enhanced MRI in the liver. Magn. Reson. Imaging 38, 47–53 (2017).
19. Le Bihan, D. What can we see with IVIM MRI? Neuroimage 187, 56–67 (2019).
20. Guo, Z., Zhang, Q., Li, X. & Jing, Z. Intravoxel incoherent motion diffusion weighted MR imaging for monitoring the instantly therapeutic efficacy of radiofrequency ablation in rabbit VX2 tumors without evident links between conventional perfusion weighted images. PLoS One 10, (2015).
21. Isola, P., Zhu, J.-Y., Zhou, T. & Efros, A. Image-to-Image Translation with Conditional Adversarial Networks. in 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR) 5967–5976 (2017). doi:10.1109/CVPR.2017.632.
Figures