0225
Transrectal magnetic resonance imaging and robot- guided focal laser ablation of prostate cancer: preliminary results of a phase 2 study.1Medical Imaging, Radboudumc Nijmegen, Nijmegen, Netherlands, 2Urology, Radboudumc Nijmegen, Nijmegen, Netherlands
Synopsis
Magnetic resonance imaging-guided focal laser ablation is used as alternative to radical treatment while preserving healthy tissue and subsequently reduce treatment-related morbidity in patients with localized prostate cancer. Preliminary results of this prospective, non-randomized, clinical phase 2 study using a remote-controlled manipulator, show promising results in both oncological and functional outcomes in a group of 20 patients with low- to intermediate-risk (Gleason Score ≤ 4+3) prostate cancer. Transrectal magnetic resonance imaging-guided focal laser ablation is a promising outpatient procedure under local anesthesia for localized prostate cancer. It provides a fast and minimally-invasive strategy while reducing treatment related complications and side-effects.
Introduction
Prostate cancer (PCa) is the most frequent malignancy in the male population and has a substantial socio-economic impact (1). At present, treatment choice for patients with low- to intermediate risk of disease progression lies between active surveillance (AS) and radical therapies, i.e. radical prostatectomy or radiotherapy (2-3). Radical treatment options provide an excellent long-term cancer efficacy control but also come with treatment-related complications and side-effects, i.e. urinary incontinence, sexual dysfunction and bowel urgency (4-5). Minimally-invasive intervention options aim for targeted PCa therapy while preserving healthy tissue and subsequently reduce treatment-related morbidity (6-7). Focal laser ablation (FLA) is a thermal ablation technique using energy provided by an optical laser fiber that is applied within the cancerous tissue by either a transrectal or transperineal approach. This results in direct-focused cell death by raising the temperature of the targeted tissue above 60°C. Image-guided interventions using magnetic resonance imaging (MRI) provide improved treatment accuracy due to excellent soft-tissue contrast, multi-planar and anatomical imaging (8). Real-time feedback of the heat distribution within the prostatic tissue allows accurate focal treatment nearby adjacent structures. The use of MRI-guided FLA holds the promise to provide a faster, less expensive and less invasive alternative to radical PCa treatment (9). Despite recent studies, long-term follow-up is lacking and the efficacy and functional outcomes are not well established. This study evaluates the use of transrectal MRI-guided and robot-assisted FLA in patients with low- to intermediate risk PCa in an outpatient setting under local anesthesia.Methods
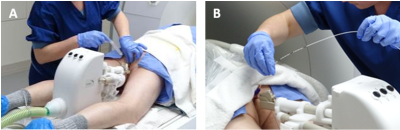
Fifty-three men of ≥45 years with newly diagnosed low- to intermediate-risk (Gleason score ≤ 4+3 or ISUP≤3) PCa and a prostate specific antigen (PSA) level ≤15 ng/mL, will undergo transrectal MRI-guided (3.0-T Skyra; Siemens Healthineers, Erlangen, Germany) FLA in a prospective, non-randomized, clinical phase 2 study. The treatment is performed as an outpatient procedure under local anesthesia. A MRI-compatible CE marked remote-controlled manipulator (RCM) (Soteria Medical, The Netherlands) is used for needle guidance towards the area of interest (figure 1). When the needle guide was targeted to the lesion, the laser fiber was introduced and the ablation was started (Leonardo laser system, Biolitec, Germany). The thermal distribution is monitored using real-time temperature mapping in two directions (axial/sagittal: TR 24 ms TE 8 ms, flip angle 15°, resolution 1.0x1.0mm, slice thickness 5.0mm). Multiple ablations are performed to cover the whole tumor (figure 2). Follow-up includes of PSA level measurement and dedicated questionnaires for functional outcome (IPSS and ICIQ for urinary outcomes, EORTC QLQ-PR25 and IIEF for sexual outcomes, ) after 6 weeks, 3,6,12,and 24 months, multiparametric prostate MR examination at 6,12 and 24 months and a targeted MR-guided biopsy of the ablation zone at 12 months after the FLA procedure. Furthermore, re-treatment percentage, success rate and complication rates were assessed during follow-up visits.Results
Thus far, twenty patients were successfully treated with MR-guided FLA. The median PSA level at baseline was 6.8 (2.7-15) ng/mL. Four patients had a Gleason 3+3 and 16 patients had a Gleason 3+4 prostate tumor. All treated lesions were located in the peripheral zone. The number of ablations varied from 3 to 6 per procedure. One patient was unable to urinate directly post-procedure and a transurethral catheter was inserted for 5 days. A second patient received a transurethral catheter 2 days after the procedure due to acute urinary retention. Despite the adequate antibiotic policy, one patient developed a fever and was admitted to the hospital for intravenous antibiotic administration in the week following the procedure as a result of an infection. No other complications were recorded. Completed two-year follow-up is known for the first nine patients. The mean (range) PSA level at 6, 12 and 24 months after treatment were 4.4 (0.56-16.1) ng/mL, 3.8 (0.36-8.1) ng/mL and 3.8 (0.38-9.0) ng/mL respectively. Two patients had an increased PSA level due to a second (newly developed) non-significant (Gleason Score 3+3) prostate tumor. Two other patients underwent a radical prostatectomy after initial treatment with FLA due to evidence of a newly developed significant (Gleason Score ≥3+4) tumor on MRI and histopathology during follow-up. The multiparametric prostate MRI and subsequent targeted biopsies of all other patients showed no evidence of recurrent or residual disease during follow-up. There were no significant changes in functional outcome and quality of life.Discussion
This research shows the pre-liminary results of a prospective phase-2 study. The PSA levels decreased severely and the majority of the treated patients had no presence of significant recurrent or residual tumor on multi-parametric MRI or in the histopathology results during follow-up. Furthermore, none of the patients showed significant changes or deterioration of functional outcome measurements and quality of life. Despite the promising results, this study is limited by its single-center and longitudinal study design, which may lead to a selection bias. All included patients were widely counseled for other (definitive) treatment options or active surveillance.Conclusion
Transrectal robot-assisted MRI-guided FLA provides promising early results in oncological control and effectiveness without significant changes in functional outcome and quality of life in patients with low- to intermediate-risk PCa.Acknowledgements
No acknowledgement found.References
- Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2017; 71: 618–29.
- Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst 2006; 98: 715–7.
- Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008; 358: 1250–61.
- Resnick MJ, Koyama T, Fan K-H, et al. Long-Term functional outcomes after treatment for localized prostate cancer. N Engl J Med 2013; 368: 436–45.
- Eggener S, Salomon G, Scardino PT, et al. Focal therapy for prostate cancer: possibilities and limitations. Eur Urol 2010; 58: 57–64.
- Valerio M, Ahmed HU, Emberton M, et al. The role of focal therapy in the management of localized prostate cancer: a systematic review. Eur Urol 2014; 66: 732–51.
- Bomers JGR, Sedelaar JPM, Barentsz JO, et al. Mri-Guided interventions for the treatment of prostate cancer. AJR Am J Roentgenol 2012; 199: 714–20.
- van Luijtelaar A, Greenwood BM, Ahmed HU, et al. Focal laser ablation as clinical treatment of prostate cancer: report from a Delphi consensus project. World J Urol 2019; 37: 2147–53.
Figures
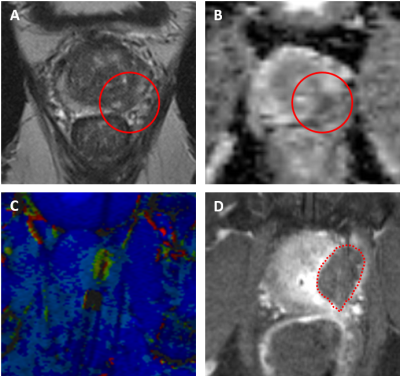
Magnetic resonance imaging of a 65-year-old male with an initial prostate specific antigen level of 3.9 ng/mL that underwent transrectal robot-assisted focal laser ablation for a de novo prostate cancer (Gleason Score 3+3) at the left peripheral zone (PI-RADS 5) in the mid prostate. (A) Tumor (red circle) on axial T2-weighted imaging; (B) Lesion (red circle) on axial apparent diffusion coefficient (ADC)map with a ADC value of 645; (C) Intra-procedural thermometry imaging. (D) Axial contrast enhanced T1-weighted images after treatment shows the ablation zone (red dotted line).