0224
A multimodality, MRI positive-contrast fiducial marker for prostate cancer treatment: first-in-patient experience with proton therapy1Imaging Physics, UT MD Anderson Cancer Center, Houston, TX, United States, 2Experimental Radiation Oncology, UT MD Anderson Cancer Center, Houston, TX, United States, 3Radiation Physics, UT MD Anderson Cancer Center, Houston, TX, United States, 4Radiation Oncology, UT MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Carbon and gold fiducial markers (FMs) are the most commonly used FMs for prostate radiotherapy. However, these markers do not produce positive contrast on MRI, and can cause large magnetic susceptibility artifacts on MRI. These properties of carbon and gold FMs make their identification on MRI challenging and complicate treatment planning processes that use co-image registration of MRI with CT for radiotherapy treatments. A novel multimodality FM has been developed that produces positive contrast on four imaging modalities, including MRI. This work presents the first-in-patient experience using the multimodality FM for proton therapy of prostate cancer.
Introduction
Radiation therapy (RT) is a standard of care treatment option for prostate cancer. Fiducial markers (FMs) implanted into the prostate aid with patient positioning [1]. The implanted FMs are identified under image guidance to position the patient in the appropriate orientation for treatment delivery.Carbon and gold FMs are the most commonly used FMs for prostate radiotherapy. Carbon and gold markers appear as regions of positive contrast on ultrasound (US) and imaging modalities using X-rays, such as computed tomography (CT) and kV imaging, due to their X-ray attenuation properties. However, these markers do not produce positive contrast on MRI, and can cause large magnetic susceptibility artifacts on MRI. These properties of carbon and gold FMs make their identification on MRI challenging and complicate the process of treatment planning using co-image registration of MRI with CT.
This work investigates a novel multimodality fiducial marker (MMFM) (NOVA, C4 Imaging, Houston, TX) for radiation treatments of prostate cancer with MRI. The first patient experience imaging the MMFM for treatment planning and treatment set up is presented.
Methods
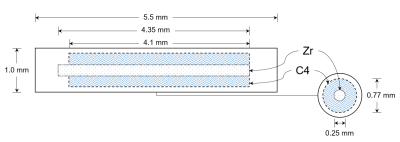
Multimodality fiducial markerThe MMFM is constructed of a zirconium core surrounded by a cobalt dichloride-N-acetyl cysteine (C4) contrast solution (Figure 1). The zirconium and contrast agent are encapsulated by polyether ether ketone. The physical length of the MMFM is 5.5 mm, and the physical diameter is 0.8 mm. The zirconium core has a length of 4.35 mm and a diameter of 0.25 mm. The C4 solution encompasses a diameter of 0.77 mm and length of up to 4.1 mm.
The zirconium core has a higher linear attenuation coefficient than the surrounding C4 and prostate tissue providing the mechanism for positive contrast under CT and kV imaging. The C4 solution has a short T1 and provides positive contrast under T1-weighted and T2-T1-weighted MRI. As a result, the active length of the MMFM is 4.35 mm for CT and kV modalities; the active length is up to 4.1 mm for MRI.
The MMFM has demonstrated lower dose perturbations compared to carbon and gold FMs [2].
Phantom imaging
Fully balanced steady-state free precession (SSFP) MRI was previously investigated for imaging implanted seed markers for prostate brachytherapy, which were also constructed of the encapsulated C4 contrast solution [3]. This pulse sequence demonstrated high spatial resolution imaging for imaging the small seed markers, while simultaneously providing sufficient SNR and soft-tissue contrast for anatomy contouring.
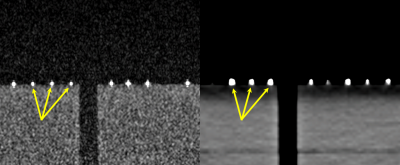
Prior to patient imaging, we tested the existing MRI protocol developed for the seed markers on the MMFMs. The MMFMs were positioned atop of an anthropomorphic Rando phantom. They were imaged with both a routine prostate protocol on CT and an existing fully balanced SSFP prostate MRI protocol [3].
Patient imaging
The first patient receiving MRI-based proton therapy was implanted with the MMFM under transrectal US guidance. Three markers were implanted into the patient’s prostate: one in the right base of the prostate, one in the left midgland of the prostate, and one in the right apex of the prostate.
The patient was imaged with a routine abdomen-pelvis protocol of the prostate at 120 kVp. The CT was interpolated to near-isotropic resolution (0.98 × 0.98 × 1 mm3). The field-of-view (FOV) was 500 mm axially; 380 slices were acquired.
A fully balanced SSFP MRI was acquired on a 3T Siemens Prisma (Siemens Healthineers, Erlangen, Germany). Scan parameters were: TR/TE = 6.72/3.36 ms; NEX = 1; RBW = 475 Hz/px; FA = 41°; FOV = 150 mm; slice thickness = 1.2 mm (72 slices); and Nx = 320.
Results
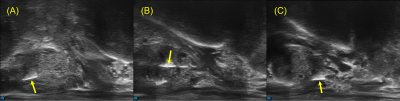
The phantom imaging validated the use of existing MRI techniques for imaging the MMFM, which appeared as regions of hyperintensity under both MRI and CT (Figure 2).The interventional radiologist implanting the MMFMs reported that deployment was identical to carbon and gold markers. The MMFMs had similar echogenicity as carbon and gold markers (Figure 3).
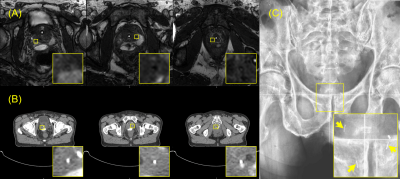
The MMFMs appeared as regions of positive contrast on all four imaging modalities (US, Figure 3; MRI, Figure 4A; CT, Figure 4B; kV Figure 4C). The measured lengths of the markers on the kV images were 4.5 mm (base), 4.8 mm (midgland), and 4.7 mm (apex); they were 6.2 mm (base), 6.5 mm (midgland), and 6.3 mm (apex) on the CT images; they were 6.5 mm (base), 6.3 mm (midgland), and 5.7 mm (apex) on the US images; finally, they were 3.83 mm (base), 3.88 mm (midgland), and 3.2 mm (apex) on the MRIs.
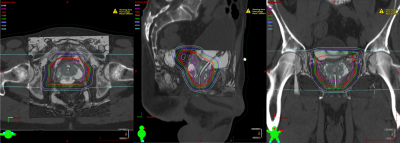
Excellent registration was observed between the CT and MRI for treatment planning (Figure 5), which was facilitated by the coregistration of the markers on the CT and MRI.
Discussion
Conventional FMs used for prostate radiotherapy provide positive contrast on US, CT, kV imaging only. To the best of our knowledge, the MMFM studied in this work is the first to provide positive contrast on MRI in addition to US, CT, and kV imaging. This first-in-patient study demonstrated the feasibility of a single FM to be imaged under four major modalities used for image-guided radiotherapy of prostate cancer.Conclusions
Multimodality fiducial markers providing positive contrast on MRI may help to incorporate MRI into radiation therapy treatments of prostate cancer.Acknowledgements
No acknowledgement found.References
[1] O’Neill AGM, et al. Fiducial marker guided prostate radiotherapy: a review. Br J Radiol. 2016;89(1068):20160296.
[2] Wang L, et al. The Dosimetric Effect of MRI Positive-Contrast Markers vs. Negative-Contrast Fiducial Markers on Proton Radiation. Int J Radiat Oncol Biol Phys. 2021; 111(3):e129-e130.
[3] Sanders J, et al. Fully Balanced SSFP Without an Endorectal Coil for Postimplant QA of MRI-Assisted Radiosurgery (MARS) of Prostate Cancer: A Prospective Study. Int J Radiat Oncol Biol Phys. 2021;109(2):614-625.
Figures