0219
Longitudinal associations between 4D flow MRI intracranial pulsatility, white matter lesions, and perivascular space dilation across 5 years1Department of Radiation Sciences, Umeå University, Umeå, Sweden, 2Umeå Center for Functional Brain Imaging (UFBI), Umeå University, Umeå, Sweden, 3Department of Clinical Science, Neurosciences, Umeå University, Umeå, Sweden, 4Department of Integrative Medical Biology (IMB), Umeå University, Umeå, Sweden, 5Department of Applied Physics and Electronics, Umeå University, Umeå, Sweden
Synopsis
Small vessel disease (SVD) is associated with elevated vascular stiffness and pulsatility, but longitudinal studies are lacking. Using a 4D flow MRI approach sensitive to age-differences in pulsatility in large and small cerebral arteries, we evaluated 5-year changes in pulsatility in relation to changes in white matter lesions (WML) and perivascular spaces (PVS), radiological markers of SVD. Pulsatility change correlated with WML and PVS volume change, but pulsatility at baseline did not predict WML and PVS progression. However, WML and PVS volumes at baseline predicted 5-year pulsatility change, suggesting that microvascular dysfunction associated with SVD accelerates pulsatility increases.
Introduction
Small vessel disease (SVD) is characterized by dysfunction in cerebral microvessels and is present in approximately half of dementia cases1–3. Manifestations of SVD can be observed on MRI as white matter lesions (WML), dilated perivascular spaces (PVS), microbleeds, microinfarcts, and lacunar infarcts1–3. Vascular stiffness and excessive blood flow pulsatility could be involved in the pathogenesis of SVD4–6; however, current evidence is almost exclusively cross-sectional. Conclusions about how ongoing disease processes are related in the incipient stages of brain aging require population-based cohorts following individuals over time. We have developed 4D flow MRI post-processing methods for pulsatility assessment in major7,8 and distal cerebral arteries9 and found cross-sectional associations to age9 and brain function10. Here we apply this methodology to investigate longitudinal relationships between pulsatility, WML volume, and PVS volume across 5 years of normal aging.Methods
4D flow and structural MRI data were collected in the the Cognition, Brain, and Aging (COBRA) study, a population-based prospective study on aging (N=181, ages: 64-68 years; MMSE 27+)11. Participants underwent baseline assessments during 2012-2014, and N=129 returned for follow up 5 years later. The study was conducted in accordance with the Declaration of Helsinki and approved by the Regional Ethical board of Umeå, Sweden. Written informed consent was provided from all participants prior to any testing. 4D flow MRI was performed with the PC-VIPR sequence that provides flow velocities in all spatial directions, time-resolved over the cardiac cycle, with whole-brain coverage12,13. Pulsatile flow waveforms were sampled from the internal carotid arteries (ICA) and for small, distal cerebral arteries9 along automatically segmented centerline structures7. Seed points were manually placed in the ICAs, and distal cerebral arteries were identified from veins and large arteries using a previously described post-processing method9. Waveform-averaging along the identified vessel segments were then used to estimate a single ICA and a single distal arterial waveform (Figure 1), that were characterized by the pulsatility index (PI). Structural MRI included T1-weighted, T2-weighted, and FLAIR. SPM12 and a lesion-growth algorithm was used to segment total WML volume from T1-weighted and FLAIR volumes14. T1- and T2-weighted volumes were used to estimate a quotient volume (T2/T1), where dilated PVS display high intensities15. Visualization was further improved with a vessel enhancement filter16. Total PVS volume was estimated as the sum of PVS volumes in basal ganglia, white matter, and brainstem. Linear mixed-effects models (LMEs) were used to estimate age-related changes. Participant-specific intercepts and slopes from LMEs were used as baseline and change variables to test associations between pulsatility, WML, and PVS.Results
5-year increases were found for PI in ICA (Δ=0.062; p=1e-06) and distal (Δ=0.052; p=6e-08) arteries, indicating that the 4D flow MRI pulsatility measures were highly age-sensitive. 5-year increases were also found for WML (Δ=1.67ml; p=4e-21) and PVS (Δ=4.52ml; p=6e-21) volumes. Larger increases in PI were related to more rapid WML and PVS progression (Figure 2a). Similar associations were found for distal arteries (r=0.23; p=0.019 for WML; r=0.28; p=0.0065 for PVS) and ICA (r=0.21; p=0.036 for WML; r=0.22; p=0.034 for PVS). To investigate whether PI increases precede SVD progression, or arise as a consequence of the disease process, we then explored baseline – change associations. In contrast to our expectations, baseline PI did not predict WML (p=0.53 for ICA; p=0.71 for distal PI) or PVS (p=0.18 for ICA; p=0.53 for distal PI) progression (Figure 2b). However, WML and PVS volumes at baseline were associated with PI increases (Figure 2c). Again, observations were similar for distal arteries (r=0.28; p=0.0047 for WML; r=0.24; p=0.015 for PVS) and ICA (r=0.21; p=0.034 for WML; r=0.20; p=0.037 for PVS).Discussion
4D flow MRI and comprehensive post-processing methods were effective for detecting age-relates changes in pulsatility across 5 years. This study is the first to evaluate correlated changes in pulsatility and SVD markers, and test the possibility that SVD lesions may precede pulsatility increases. SVD is characterized by wall thickening, tortuosity, and lumen narrowing of the vessels that supply white matter1,2. Our results suggest that such SVD related changes precede the observed pulsatility increases. Previous longitudinal studies used ultrasound and only investigated whether baseline pulsatility could predict WML progression17,18. Our findings agree with a study where PI at baseline failed to predict WML volume increases over five years18. Our findings do not rule out that pulsatility could harm other parts of the brain. For example, the hippocampus is supplied by short arterial branches that provide little damping-capacity. This could explain why pulsatility has been observed to correlate negatively with hippocampal volumes19,20 and episodic memory10. In animal21 and cell-culture22 studies, excessive pulsatility trigger blood-brain barrier (BBB) breakdown. Hence, pulsatility be may particularly interesting to study in relation to hippocampal BBB breakdown23–25.Conclusions
4D flow MRI derived pulsatility of both large and small cerebral arteries were highly sensitive to aging. Increases in pulsatility correlated with progression of WML and PVS volumes in a population-based cohort. Further, baseline WML and PVS volumes predicted 5-year changes in pulsatility, whereas baseline pulsatility failed to predict WML and PVS progression. Hence, our findings do not support pulsatility as a contributing mechanism in the incipient stages of SVD. Instead, our observations suggest that vascular changes associated with SVD accelerate age-related increases in pulsatility.Acknowledgements
The COBRA project was funded by grants from the Swedish Research Council (grant number: 421-2012-648 and grant number: 2017-02217). This study was further supported by grants from the Knut and Alice Wallenberg Foundation, a donation of the Jochnick Foundation, grants from the Swedish Foundation for Strategic Research and grants from the Västerbotten County Council and the Swedish Research Council (grant number: 2017- 04949).References
1. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701.
2. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 2019; 18: 684–696.
3. Wardlaw JM, Benveniste H, Nedergaard M, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 2020; 16: 137–153.
4. Webb AJS, Simoni M, Mazzucco S, et al. Increased cerebral arterial pulsatility in patients with leukoaraiosis: Arterial stiffness enhances transmission of aortic pulsatility. Stroke 2012; 43: 2631–2636.
5. Shi Y, Thrippleton MJ, Marshall I, et al. Intracranial pulsatility in patients with cerebral small vessel disease: a systematic review. Clin Sci (Lond) 2018; 132: 157–171.
6. Pahlavian SH, Wang X, Ma S, et al. Cerebroarterial pulsatility and resistivity indices are associated with cognitive impairment and white matter hyperintensity in elderly subjects : A phase-contrast MRI study. J Cereb Blood Flow Metab 2020; 0271678X20927101.
7. Schrauben E, Ambarki K, Spaak E, et al. Fast 4D flow MRI intracranial segmentation and quantification in tortuous arteries. J Magn Reson Imaging 2015; 42: 1458–1464.
8. Dunås T, Holmgren M, Wåhlin A, et al. Accuracy of blood flow assessment in cerebral arteries with 4D flow MRI: Evaluation with three segmentation methods. J Magn Reson Imaging 2019; 50: 511–518.
9. Vikner T, Nyberg L, Holmgren M, et al. Characterizing pulsatility in distal cerebral arteries using 4D flow MRI. J Cereb Blood Flow Metab 2020; 40: 2429–2440.
10. Vikner T, Eklund A, Karalija N, et al. Cerebral arterial pulsatility is linked to hippocampal microvascular function and episodic memory in healthy older adults. J Cereb Blood Flow Metab 2021; 0271678X20980652.
11. Nevalainen N, Riklund K, Andersson M, et al. COBRA: A prospective multimodal imaging study of dopamine, brain structure and function, and cognition. Brain Res 2015; 1612: 83–103.
12. Gu T, Korosec FR, Block WF, et al. PC VIPR: a high-speed 3D phase-contrast method for flow quantification and high-resolution angiography. AJNR Am J Neuroradiol 2005; 26: 743–749.
13. Johnson KM, Markl M. Improved SNR in phase contrast velocimetry with five-point balanced flow encoding. Magn Reson Med 2010; 63: 349–355.
14. Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage 2012; 59: 3774–3783.
15. Schwartz DL, Boespflug EL, Lahna DL, et al. Autoidentification of perivascular spaces in white matter using clinical field strength T(1) and FLAIR MR imaging. Neuroimage 2019; 202: 116126.
16. Jerman T, Pernus F, Likar B, et al. Enhancement of Vascular Structures in 3D and 2D Angiographic Images. IEEE Trans Med Imaging 2016; 35: 2107–2118.
17. Lee W-J, Jung K-H, Ryu YJ, et al. Progression of Cerebral White Matter Hyperintensities and the Associated Sonographic Index. Radiology 2017; 284: 824–833.
18. Kneihsl M, Hofer E, Enzinger C, et al. Intracranial Pulsatility in Relation to Severity and Progression of Cerebral White Matter Hyperintensities. Stroke 2020; 51: 3302–3309.
19. Wåhlin A, Ambarki K, Birgander R, et al. Intracranial pulsatility is associated with regional brain volume in elderly individuals. Neurobiol Aging 2014; 35: 365–372.
20. Miller ML, Ghisletta P, Jacobs BS, et al. Changes in cerebral arterial pulsatility and hippocampal volume: a transcranial doppler ultrasonography study. Neurobiol Aging 2021; 108: 110–121.
21. De Montgolfier O, Pinçon A, Pouliot P, et al. High Systolic Blood Pressure Induces Cerebral Microvascular Endothelial Dysfunction, Neurovascular Unit Damage, and Cognitive Decline in Mice. Hypertension 2019; 73: 217–228.
22. Garcia-Polite F, Martorell J, Del Rey-Puech P, et al. Pulsatility and high shear stress deteriorate barrier phenotype in brain microvascular endothelium. J Cereb Blood Flow Metab 2017; 37: 2614–2625.
23. Nation DA, Sweeney MD, Montagne A, et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 2019; 25: 270–276.
24. Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature 2020; 581: 71–76.
25. Wåhlin A, Nyberg L. At the Heart of Cognitive Functioning in Aging. Trends Cogn Sci 2019; 23: 717–720.
Figures
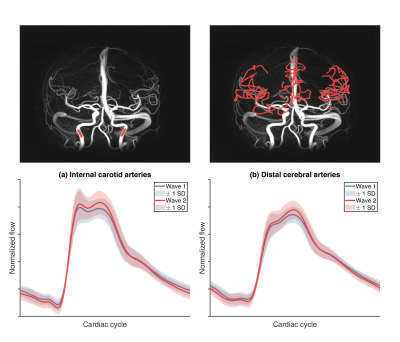
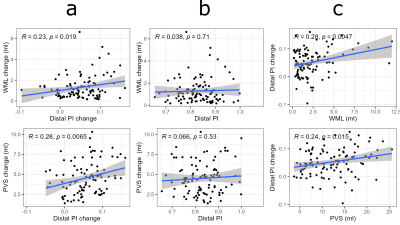
Figure 2. Distal arterial pulsatility index (PI) in relation to white matter lesion (WML) and perivascular space (PVS) volumes. (a) Longitudinal change – change associations across 5 years. (b) Baseline PI in relation to 5-year changes in WML and PVS volumes. (c) WML and PVS volumes at baseline in relation to 5-year changes in PI. Baseline and change correspond to participant-specific intercepts and slopes from linear mixed-effects models. The reported coefficients were obtained by Pearson correlation. Note: similar observations were found for the internal carotid arteries.