0215
Free-running 3D anatomical and flow MRI using Synchronization of Neighboring Acquisitions by Physiological Signals (SyNAPS)1Department of Diagnostic and Interventional Radiology, University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Woman- Mother- Child Department, University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 4Service of Cardiology, Centre de Resonance Magnétique Cardiaque (CRMC), University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 5Department of Diagnostic, Interventional and Pediatric Radiology (DIPR), University hospital Bern (Inselspital), Bern, Switzerland, 6Translational Imaging Center, sitem-insel, Bern, Switzerland, 7Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 8Department of Biomedical Engineering, Northwestern University, Chicago, IL, United States, 9Center for Biomedical Imaging (CIBM), Lausanne, Switzerland
Synopsis
We introduce a novel method for combining multiple free-running MRI acquisitions together, through the use of cardiac and respiratory signal extraction with Pilot Tone navigation called Synchronization of Neighboring Acquisitions by Physiological Signals (SyNAPS). We demonstrate the initial feasibility and utility of SyNAPS on a setup for joint reconstruction of back-to-back dynamic anatomical and flow MRI acquisitions, here named 4D flow SyNAPS. Overall, 4D flow SyNAPS enabled an improved structural visualization, when compared to the magnitude images from free-running 4D flow datasets alone, and the resulting flow measurements showed better agreement with reference 2D flow acquisitions.
Introduction
The free-running framework was recently developed for fully self-gated whole-heart MRI [1] and has been extended to angiography [2], flow [3,4], T1 [5] and fat fraction [6] mapping. Each of these branches benefit from a simplified workflow and predictable scan times without the need for ECG gating or respiratory navigation. However, self-gating, which extracts cardiac and respiratory motion signals from the data themselves, has been shown to have unpredictable shifts relative to known physiological markers (i.e. R-wave, end-expiratory position), precluding a comprehensive analysis of different free-running branches in the same exam, or requiring additional manual spatial and temporal synchronization of the resulting images [1]. In this work, we present a novel approach for combining multiple free-running acquisitions called Synchronization of Neighboring Acquisitions by Physiological Signals (SyNAPS). In the proposed SyNAPS framework, we use the Pilot Tone (PT) navigation system [4,7,8] to acquire cardiac and respiratory motion signals in parallel to sequential free-running acquisitions. The PT signals, fully independent from the imaging acquisitions, then inform a joint respiratory motion-corrected and cardiac motion-resolved 4D image reconstruction [9,10]. Here, we demonstrate the initial feasibility and utility of SyNAPS using sequentially acquired free-running 3D radial fast interrupted steady-state (FISS) [11] and free-running 3D radial flow (4D Flow) [3,10] datasets. We test the hypothesis that using SyNAPS, the magnitude images from FISS can be leveraged to improve vessel segmentation and subsequent flow quantification in the free-running 4D Flow data [12]. We compare this approach called 4D Flow SyNAPS to 4D Flow alone, and to a 2D Flow reference standard.Methods
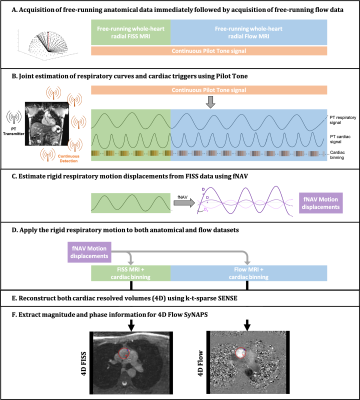
Five healthy volunteers (2F, ages 23-32) and two Marfan Syndrome patients (2F, ages 14-18) were scanned on a 1.5T MAGNETOM Sola system (Siemens Healthcare, Erlangen, Germany) using a 12-channel body coil array with an integrated PT transmitter. All subjects provided written informed consent compliant with our institutional guidelines and approved by the local research ethics committee. Two 2D Flow datasets were acquired as reference (ascending and descending aorta (AAo, DAo) (TR/TE=5.1/2.9ms, venc=150cm/s, FOV=380x260mm2, spatial resolution=2.0x2.0x6.0mm3, Scan time=0:15min). Then, two prototype free-running radial whole-heart MRI sequences [1] were ran, the first one was FISS [11] followed immediately by 4D Flow [3,10] (Figure 1A). Scan parameters for FISS were TR/TE=2.94/1.5ms, segments=12000, readouts per FISS module=4, number of FISS modules=6, FOV=(220mm)3, spatial resolution=(2.0mm)3, Scan time=3:45min. Scan parameters for 4D Flow were TR/TE=5.3/3.5ms, shots=4820, segments=21, venc=150cm/s, FOV=(220mm)3, spatial resolution=(2.0mm)3, Scan time=8:59min. SyNAPS was used to connect the reconstruction of the two sequences (Figure 1B-E). PT respiratory and cardiac signals spanning the two free-running sequences were extracted for subsequent respiratory motion correction and cardiac binning (Figure 1B). Translational respiratory motion correction of the underlying k-space data was performed on both free-running FISS and 4D flow datasets, using focused navigation (fNAV) coefficients estimated from the FISS data (Figure 1C-D) [9,10]. Finally, each dataset was reconstructed into cardiac motion-resolved volumes (4D) using a k-t-sparse SENSE algorithm (Figure 1E-F). To demonstrate the utility of the SyNAPS framework, the high blood-myocardium contrast images from FISS were combined with the phase images from 4D Flow to create 4D Flow SyNAPS. This was compared to 4D Flow alone, as well as to the reference 2D Flow data by retrospectively extracting matching slice positions. Measurements in the AAo and DAo were quantitatively compared (Circle cvi42, Calgary, Canada), in terms of flow measurements (flow rate, net volume, peak flow) and vessel area over the cardiac cycle.Results
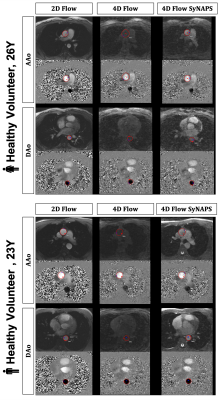
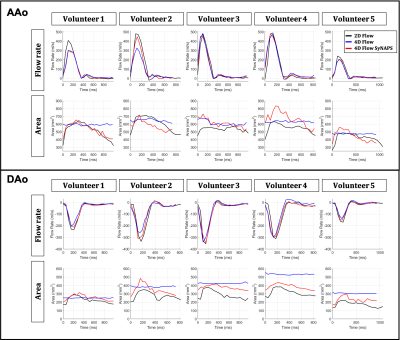
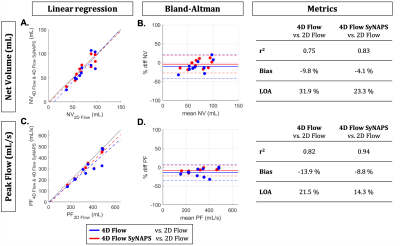
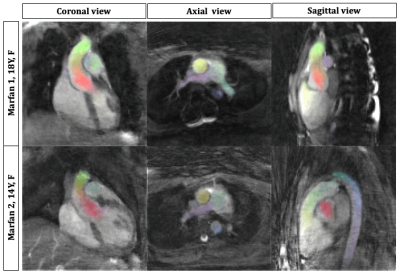
The contrast of 4D Flow SyNAPS magnitude images (derived from the FISS sequence) demonstrates a clear improvement over 4D Flow alone (Figure 2), and are comparable to the 2D Flow images, which benefit from in-flow enhancement. For all five healthy volunteers, 4D Flow SyNAPS yielded flow rates and vessel area changes comparable to 2D Flow MRI (Figure 3). Linear regression reported similar significant correlation between all flow datasets (p<0.05); Bland-Altman analysis reported a lower bias and limits of agreement between 4D Flow SyNAPS and 2D Flow (Figure 4) relative to 4D Flow alone. For the two patient datasets, fusion of anatomical and flow information (Figure 5) clearly demonstrates the successful synchronization of the two sequences.Discussion and Conclusion
This work introduces SyNAPS, a framework that builds towards comprehensive whole-heart MRI by synchronizing different branches of the free-running framework. We demonstrated the initial feasibility and utility of SyNAPS on a setup for joint whole-heart anatomical and flow MRI that does not require ECG gating or respiratory navigators. We show that the high-contrast anatomical imaging sequence can be leveraged to improve 3D flow measurements that often suffer from poor delineation of the vessel boundaries in the absence of contrast agents [12]. These promising initial results motivate further validation of the framework, especially in the context of heart-rate variability and respiratory drift. While the current implementation used the respiratory signal for motion correction, this framework could be easily extended to respiratory-resolved 5D imaging. Finally, SyNAPS can be readily applied to other branches of the free-running framework in order to create a simplified workflow for a comprehensive assessment of the structure, dynamic function, blood flow, and tissue properties of the heart, with the overarching goal of creating new MRI-based tools in the diagnosis and management of heart disease.Acknowledgements
The present study was funded by the Swiss National Science Foundation, SNSF (173129, 320030B_201292, PCEFP2_194296, PZ00P3_167871, 32003B_182615).References
1. Di Sopra L, Piccini D, Coppo S, Stuber M, Yerly J. An automated approach to fully self‐gated free‐running cardiac and respiratory motion‐resolved 5D whole‐heart MRI. Magn Reson Med. 2019;1–15.
2. Heerfordt J, Whitehead KK, Bastiaansen JAM, Di Sopra L, Roy CW, Yerly J, et al. Similarity-driven multi-dimensional binning algorithm (SIMBA) for free-running motion-suppressed whole-heart MRA. Magn Reson Med. 2021;86:213–29.
3. Ma LE, Yerly J, Piccini D, Sopra L Di, Roy CW, Carr JC, et al. 5D Flow MRI : A Fully Self-gated, Free-running Framework for Cardiac and Respiratory Motion – resolved 3D Hemodynamics. Radiol Cardiothorac Imaging. 2020;2(6).
4. Falcão MBL, Di Sopra L, Ma L, Bacher M, Yerly J, Speier P, et al. Pilot tone navigation for respiratory and cardiac motion-resolved free-running 5D flow MRI. Magn Reson Med. 2021;00:1–15.
5. Di Sopra L, Roy CW, Bastiaansen JAM, Yerly J, Piccini D, Arn L, et al. Fully Self-Gated Cardiac and Respiratory Motion-Resolved Isotropic 5D T1 Mapping of the Heart : Preliminary Results. Proc Intl Soc Mag Reson Med. 2019;27.
6. Mackowiak ALC, Roy CW, Yerly J, Sopra L Di, Falcão MBL, Bacher M, et al. Whole-Heart Motion-Resolved Multi-Peak Fat-Fraction Mapping using Free-Running 3D Radial Multi-Echo GRE and Pilot Tone. Proc Intl Soc Mag Reson Med. 2021;29.
7. Speier P, Fenchel M, Rehner R. PT-Nav: A Novel Respiratory Navigation Method for Continuous Acquisitions Based on Modulation of a Pilot Tone in the MR-Receiver. Proc ESMRMB. 2015. p. 129:97-98.
8. Vahle T, Bacher M, Rigie D, Fenchel M, Speier P, Bollenbeck J, et al. Respiratory Motion Detection and Correction for MR Using the Pilot Tone: Applications for MR and Simultaneous PET/MR Examinations. Invest Radiol. 2020;55:153–9.
9. Roy CW, Heerfordt J, Piccini D, Rossi G, Pavon AG, Schwitter J, et al. Motion compensated whole-heart coronary cardiovascular magnetic resonance angiography using focused navigation (fNAV). J Cardiovasc Magn Reson. BioMed Central; 2021;23:1–17.
10. Falcão MBL, Rossi GMC, Ma L, Heerfordt J, Piccini D, Yerly J, et al. Correcting vs resolving respiratory motion in accelerated free-running whole-heart radial Flow MRI using focused navigation (fNAV). Proc Intl Soc Mag Reson Med. 2021;29.
11. Bastiaansen JAM, Piccini D, Sopra L Di, Roy CW, Heerfordt J, Edelman RR, et al. Natively fat‐suppressed 5D whole‐heart MRI with a radial free‐running fast‐interrupted steady‐state ( FISS ) sequence at 1.5T and 3T. Magn Reson Med. 2019;00:1–11.
12. Jarvis KB, Wu C, Giri S, Schnell S, Barker AJ, Collins JD, et al. Improved assessment of aortic 3D blood flow with combined k-t accelerated 3D CINE bSSFP & 4D flow MRI. J Cardiovasc Magn Reson. 2016;18:1–2.
Figures