0214
Motion-Corrected 4D-Flow for Neurovascular Applications1University of Wisconsin, Madison, Madison, WI, United States
Synopsis
Accurate and precise assessment of neurovascular flow in the brain requires high image fidelity which can be difficult to obtain in populations with high tendencies to move, such as geriatrics and pediatrics. This study presents a 3D radial sampling and multi-scale low rank image reconstruction for self navigated motion correction of 4D-Flow with validations in phantom and invivo studies.
Introduction:
4D-Flow has emerged as a powerful tool to understand vascular contributions to dementia, to characterize blood flow patterns within aneurysms and arteriovenous malformations, and quantify flow features related to atherosclerosis. Further, emerging paradigms are being developed to provide unique measures of low frequency flow oscillations (LFOs)1, pulse wave velocity measures of arterial stiffness2, and flow turbulence and disorder within the neurovasculature. However, these new applications and the need to characterize flow in small vessels require 4D-Flow methods with high spatial and temporal resolution. High spatiotemporal resolution requires longer scan times that can result in increased artifact from patient motion. 3D radial trajectories are inherently less sensitive to motion and result in image blurring rather than ghosting and have previously been combined with 3D self-navigation for motion correction in T1-weighted imaging3. Self-navigation with 4D-Flow presents challenges due to increased k-space undersampling from the longer TRs and the different velocity encodings. This work uses a multi-scale low rank regularization for higher fidelity navigators4. In this work, we develop a neurovascular 4D-Flow imaging paradigm which provides motion correction capabilities. Results are demonstrated in phantom experiments and retrospectively re-analysis of human data from ongoing aging research studies.Methods:
The proposed 4D-Flow methodology (Figure 1) is based on the combination of 3D radial sampling and 3D self-navigation3. Data is collected with 3D radial sampling using pseudorandom view ordering such that the data can be binned temporally and with respect to the cardiac cycle5. Images are first reconstructed using the Extreme MRI4, multi-scale low rank framework, providing 3D temporal images with a ~1s frame rate which are rigidly registered though time to provide estimates of translation and rotation through time. These are fed into a motion corrected iterative image reconstruction, which produces motion corrected 4D-Flow data. All methods were implemented in GPU accelerated python (SigPy and PyTorch) including integrated registration. Phantom data were acquired to validate the proposed approach. A phantom consisting of a straight silicone tube was imaged at 3.0T while pulsatile flow was driven by a pump outside the scan room (CompuFlow 1000 MR, Shelly). 4D-Flow scans were acquired with 0.7mm isotropic resolution, whole brain coverage, TE/TR=2.7/7.8ms, 338s scan time, and Venc=80cm/s. A total of four scans were acquired for which different frequencies and magnitude of motion were manually induced including: no motion, motion once during the scan, motion every 30s, and every 15s. Motion was generated by manual displacement of a table supporting the phantom setup. Time-average data were reconstructed with and without motion correction for each of the experiments.In-vivo evaluation was performed from neurovascular 4D-Flow data from 10 human volunteers from ongoing aging studies that used the same 4D-Flow protocol described for phantom experiments. Cases were selected based on low image quality of an unknown origin. Images were reconstructed using the motion corrected approach and compared to uncorrected data. All images were retrospectively reconstructed to the cardiac-cycle (20 frames) and corrected for background phase errors and velocity aliasing6. Cerebral vessels were automatically segmented7 from velocity data, and hemodynamic markers were extracted from various vessel segments including the internal carotid arteries (ICAs) and middle cerebral arteries (MCAs). 4D-Flow markers included blood flow rates, blood flow pulsatility ([Qmax-Qmin]/Qmean), and cross-sectional areas. Statistical differences in these markers were assessed using paired Student's t-test (P<0.05 significance).
Results:
Figure 2 shows phantom experiment results using this framework. Motion estimates demonstrated both obvious motion but also small levels of motion. The incorporation of motion correction into the framework substantially improves image quality in phantom scans where motion was present. Figure 3 summarizes the head motion estimates from 10 human volunteers. All cases had motions on the order of a few millimeters, indicating motion to be a reason for poor image quality. Figure 4 shows the motion correction performance on the top three cases with the largest motion in Fig 3. Overall, reduced blurring and increased vessel conspicuity were observed. Finally, figure 5 shows a significant reduction in blood flow rates, flow pulsatility index, and cross-sectional area for most vessel segments after motion correction. In addition, measurement variance and bilateral differences were notably reduced.Discussion and Conclusions:
The proposed method represents a promising approach to reduce the variance from motion in 4D-Flow MRI. Further ongoing work is needed to validate and quantify these improvements, and evaluate their potential to enable imaging in other challenging populations such as children as well during motion challenges.Acknowledgements
We gratefully acknowledge research support from GE Healthcare, and funding support from the Alzheimer's Association (AARFD-20-678095) and from NIH grants R01NS066982, P30AG062715, and R01AG021155.
References
1. Rivera-Rivera LA, Cody KA, Rutkowski D, et al. Intracranial vascular flow oscillations in Alzheimer’s disease from 4D flow MRI. NeuroImage: Clinical 2020; 28: 102379.
2. Rivera-Rivera LA, Cody KA, Eisenmenger L, et al. Assessment of vascular stiffness in the internal carotid artery proximal to the carotid canal in Alzheimer's disease using pulse wave velocity from low rank reconstructed 4D flow MRI. J Cereb Blood Flow Metab. 2021 Feb;41(2):298-311.
3. Kecskemeti S, Samsonov A, Velikina J, et al. Robust Motion Correction Strategy for Structural MRI in Unsedated Children Demonstrated with Three-dimensional Radial MPnRAGE. Radiology. 2018 Nov;289(2):509-516. doi: 10.1148/radiol.2018180180. Epub 2018 Jul 31. PMID: 30063192; PMCID: PMC6192848.
4. Ong F, Zhu X, Cheng JY, et al. Extreme MRI: Large-scale volumetric dynamic imaging from continuous non-gated acquisitions. Magn Reson Med. 2020 Oct;84(4):1763-1780. doi: 10.1002/mrm.28235.
5. Johnson KM, Lum DP, Turski PA, et al. Improved 3D Phase Contrast MRI with Off-resonance Corrected Dual Echo VIPR. Magn Reson Med. 2008 Dec; 60(6): 1329–1336.
6. Loecher M, Schrauben E, Johnson KM, et al. Phase unwrapping in 4D MR flow with a 4D single-step laplacian algorithm. J Magn Reson Imaging 2016; 43: 833–842.
7. Schrauben E, Wåhlin A, Ambarki K, et al. Fast 4D flow MRI intracranial segmentation and quantification in tortuous arteries. J Magn Reson Imaging. 2015 Nov;42(5):1458-64.
Figures
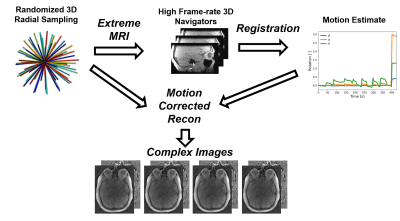
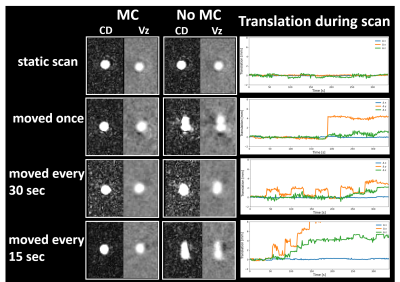
Figure 2. Flow phantom experiments showing the effects of motion correction (MC) on complex difference and velocity data for various degrees of motion. Four 4D-Flow scans were acquired on a straight silicone tube pulsatile pump driven flow phantom. For each scan, different frequencies and magnitudes of motions were induced: no motion, motion once during the scan, motions every 30s and 15s. Image quality was restored to the static scan levels after applying MC.
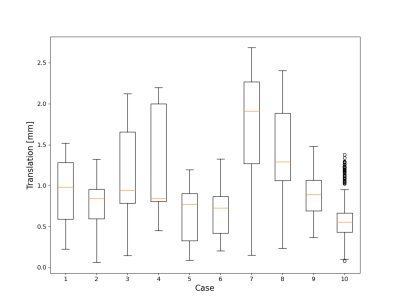
Figure 3. Summary of head motion in 10 human volunteers selected from ongoing aging research studies. 3D self-navigation from 256 time-resolved images was used to track motion, extract motion fields and correct kspace data.
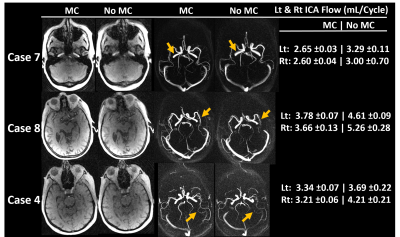
Figure 4. Exemplary images showing subjects with largest translational motion (Fig3) before and after motion correction (MC). Magnitude and complex difference images showed improved image quality including reduced vessel blurring and increased vessel conspicuity after MC. In addition, left and right internal carotid artery (ICA) blood flow magnitude, variance and bilateral differences were also reduced after MC.
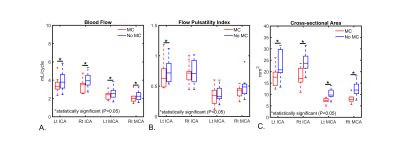
Figure 5. Summary of 4D-Flow hemodynamic markers in various cerebral vessels before and after motion correction (MC) in 10 human volunteers. Measurements were performed in the left and right internal carotid arteries (ICAs) and middle cerebral arteries (MCAs). Blood flow and cross-sectional area were significantly reduced (P<0.05) for all vessel segments after MC. Flow pulsatility index was also reduced for all vessel segments and significantly for the Left ICA (P=0.015). In addition, measurement variance, and bilateral differences were reduced after MC.