0210
Associations between cerebral microvascular hemodynamics and white matter lesion burden in typically aging older adults1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Radiology, Harvard Medical School, Boston, MA, United States
Synopsis
Hypoperfusion is frequently considered a cause of age-related white matter lesions (WML). However, reductions in oxygen availability to brain tissue may also be caused by impaired oxygen extraction fraction (OEF). Here, we tested the hypothesis that venous hyperintense signal (VHS) in arterial spin labeling (ASL) MRI is indicative of impaired OEF in typically aging older adults. In participants aged 60–80 years (n=40), we measured VHS with ASL and maximum OEF (OEFmax) with dynamic susceptibility contrast MRI. Lower OEFmax was uniquely associated with higher WML volume in participants with VHS, indicating a potentially distinct cerebrovascular aging pattern in these individuals.
Introduction
The presence of cerebral white matter lesions (WML) has been linked to declining cognitive function in typical aging as well as in patients with Alzheimer’s disease.1-7 While the etiology of these lesions is presumed to be vascular in nature,8,9 microvascular hemodynamic mechanisms underlying these lesions remain incompletely understood. Tissue hypoperfusion is frequently considered a cause of WML, but reductions in oxygen availability to the brain could also be caused by impairment in oxygen exchange efficiency. Recently, venous hyperintense signal (VHS) in arterial spin labeling (ASL) MRI has been characterized as a marker of capillary shunting and reduced OEF in young adults.10,11 VHS in perfusion-weighted ASL is thought to arise from reduced capillary transit times that allow labeled arterial blood to traverse the microvasculature without properly exchanging with tissue. Here, we tested the hypothesis that VHS in ASL MRI may also represent a general marker of impaired oxygen extraction in typically aging older adults.Methods
Participants. Older adult volunteers (60–80 years of age) were recruited as part of an IRB-approved study. Exclusion criteria included major neurological or psychiatric disorders, including vascular dementia or clinical stroke, as well other substantial systemic illnesses likely to confound the study.Data acquisition. Participants underwent two imaging sessions: non-contrast MRI at 3T (Siemens Biograph mMR) and contrast-enhanced MRI at 7T (Siemens Magnetom). T1-weighted MRI was acquired at 3T using a multi-echo MPRAGE sequence (TR=2530 ms; TI=1100 ms; TE=1.69 ms, 3.55 ms, 5.41 ms, and 7.27 ms; flip angle=7°; and spatial resolution=1.0×1.0×1.0 mm3). ASL MRI was acquired at 3T with pseudo-continuous labeling (total labeling duration=1500 ms and post-labeling delay=1200 ms) and without background or vascular suppression using a 2D EPI readout (slice thickness=5.0 mm; TR=4000 ms; TE=12 ms; matrix size=64×64; slices=22; field of view = 220×220 mm2; control/label pairs=40). Dynamic susceptibility contrast (DSC) MRI was acquired at 7T with gadolinium contrast and a 2D single-shot EPI readout (TR=1500 ms, TE=22 ms, flip angle=75°, slices=58, spatial resolution=1.5×1.5×1.5 mm3, nominal echo spacing=0.69 ms, GRAPPA=3, multiband=2, frames=100).
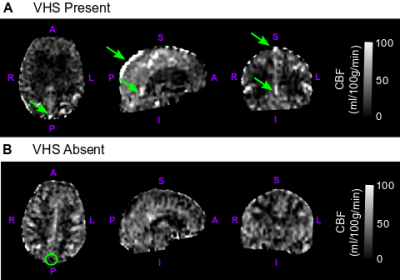
Data processing. T1-weighted images were processed to reconstruct cortical surfaces and to segment volume regions-of-interest, including gray matter and WML volume, using the Freesurfer ‘recon-all’ procedure. ASL images from each participant were pre-processed and quantified into CBF using a two-compartment kinetic model.12 CBF images from each participant were also qualitatively assessed for the presence (VHS+) or absence (VHS−) of labeled blood inside of large draining veins, including the superior sagittal sinus, inferior sagittal sinus, and straight sinus, according to a previously established procedure (Figure 1).10 DSC-MRI data were processed using the Penguin/pgui software to convert the T2* signal time curves of each voxel to contrast concentration time curves13 and used in a vascular model developed by Ostergaard et al13-15 to derive images of maximum oxygen extraction fraction (OEFmax). Average CBF and OEFmax values in global gray matter were extracted for each participant using FreeSurfer segmentations.
Results
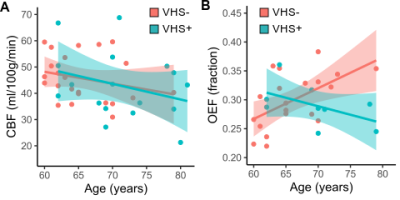
Demographics. Overall, VHS was present in 42.5% of participants (17/40). Participants with VHS (median age=70 years) were older than those without VHS (median age=64 years; p<0.01), and there were no sex differences in the groups with VHS (47% male) and without VHS (57% male; p=0.55).Cerebrovascular physiology. Older age was correlated with decreasing gray matter CBF (ρ=−0.32; p=0.05), and there was no interaction between age and VHS presence when examining the association with CBF (Figure 2A; p=0.81). There was no relationship between OEFmax and age in all participants (ρ=0.25; p=0.18), but there was a significant interaction between age and VHS presence (p<0.01). More specifically, older age is associated with higher OEFmax in participants without VHS, while older age is associated with lower OEFmax in participants with VHS (Figure 2B).
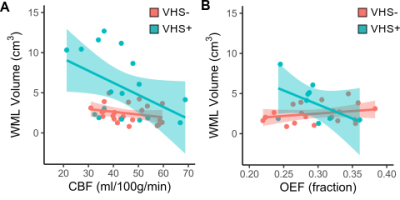
White matter lesions. WML volume was inversely correlated with CBF (ρ=−0.39; p=0.01), but there was no interaction between CBF and VHS presence for the association with WML volume (p=0.15; Figure 3A). There was no relationship between OEFmax and WML volume (ρ=−0.001; p=0.99) overall, but there was a significant interaction between OEFmax and VHS status (p=0.02). More specifically, lower OEFmax was associated with higher WML volume in participants with VHS, while higher OEFmax was associated with higher WML volume in participants without VHS (Figure 3B).
Discussion
We characterized a novel imaging marker of oxygen extraction inefficiency and its relationship with age, cerebral hemodynamic physiology, and white matter lesion burden in a cohort of older adults between 60–80 years of age. We observed that venous ASL signal indicative of capillary shunting was prevalent in 42% of older adults, and participants with VHS were older (median age=70 years) compared to participants without VHS (median age=64 years). While associated with lower CBF in both groups, the relationship between age and OEFmax was dependent on VHS presence, with lower OEFmax corresponding to older age when VHS was present. Finally, individuals with VHS exhibited greater WML burden, and higher WML volumes were correlated with both lower CBF and lower OEFmax in participants with VHS but not in those without VHS. These results indicate that the presence of VHS on perfusion-weighted ASL data could serve as a marker of a distinct cerebrovascular aging pattern that involves physiological mechanisms including oxygen extraction inefficiency in addition to hypoperfusion.Acknowledgements
This work was supported by the National Institutes of Health (R01NR010827) and the American Heart Association (19CDA34790002).References
1. Coutu JP, Goldblatt A, Rosas HD, Salat DH, (ADNI) AsDNI. White Matter Changes are Associated with Ventricular Expansion in Aging, Mild Cognitive Impairment, and Alzheimer's Disease. J Alzheimers Dis. 2016;49:329-342. doi: 10.3233/JAD-150306
2. Graff-Radford J, Arenaza-Urquijo EM, Knopman DS, Schwarz CG, Brown RD, Rabinstein AA, Gunter JL, Senjem ML, Przybelski SA, Lesnick T, et al. White matter hyperintensities: relationship to amyloid and tau burden. Brain. 2019;142:2483-2491. doi: 10.1093/brain/awz162
3. Lee S, Viqar F, Zimmerman ME, Narkhede A, Tosto G, Benzinger TL, Marcus DS, Fagan AM, Goate A, Fox NC, et al. White matter hyperintensities are a core feature of Alzheimer's disease: Evidence from the dominantly inherited Alzheimer network. Ann Neurol. 2016;79:929-939. doi: 10.1002/ana.24647
4. Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, Jack CR, Weiner M, DeCarli C, Initiative AsDN. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol. 2010;67:1370-1378. doi: 10.1001/archneurol.2010.284
5. Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192-2198. doi: 10.1212/01.wnl.0000249119.95747.1f
6. Lindemer ER, Greve DN, Fischl BR, Augustinack JC, Salat DH. Regional staging of white matter signal abnormalities in aging and Alzheimer's disease. Neuroimage Clin. 2017;14:156-165. doi: 10.1016/j.nicl.2017.01.022
7. Provenzano FA, Muraskin J, Tosto G, Narkhede A, Wasserman BT, Griffith EY, Guzman VA, Meier IB, Zimmerman ME, Brickman AM, et al. White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol. 2013;70:455-461. doi: 10.1001/jamaneurol.2013.1321
8. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689-701. doi: 10.1016/S1474-4422(10)70104-6
9. Wardlaw JM. William M. Feinberg Award for Excellence in Clinical Stroke: Small Vessel Disease; a Big Problem, But Fixable. Stroke. 2018;49:1770-1775. doi: 10.1161/STROKEAHA.118.021184
10. Juttukonda MR, Donahue MJ, Davis LT, Gindville MC, Lee CA, Patel NJ, Kassim AA, Pruthi S, Hendrikse J, Jordan LC. Preliminary evidence for cerebral capillary shunting in adults with sickle cell anemia. J Cereb Blood Flow Metab. 2019;39:1099-1110. doi: 10.1177/0271678X17746808
11. Juttukonda MR, Donahue MJ, Waddle SL, Davis LT, Lee CA, Patel NJ, Pruthi S, Kassim AA, Jordan LC. Reduced oxygen extraction efficiency in sickle cell anemia patients with evidence of cerebral capillary shunting. J Cereb Blood Flow Metab. 2020:271678X20913123. doi: 10.1177/0271678X20913123
12. Fan AP, Guo J, Khalighi MM, Gulaka PK, Shen B, Park JH, Gandhi H, Holley D, Rutledge O, Singh P, et al. Long-Delay Arterial Spin Labeling Provides More Accurate Cerebral Blood Flow Measurements in Moyamoya Patients: A Simultaneous Positron Emission Tomography/MRI Study. Stroke. 2017;48:2441-2449. doi: 10.1161/STROKEAHA.117.017773
13. Mouridsen K, Friston K, Hjort N, Gyldensted L, Østergaard L, Kiebel S. Bayesian estimation of cerebral perfusion using a physiological model of microvasculature. Neuroimage. 2006;33:570-579. doi: 10.1016/j.neuroimage.2006.06.015
14. Jespersen SN, Østergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab. 2012;32:264-277. doi: 10.1038/jcbfm.2011.153
15. Mouridsen K, Hansen MB, Østergaard L, Jespersen SN. Reliable estimation of capillary transit time distributions using DSC-MRI. J Cereb Blood Flow Metab. 2014;34:1511-1521. doi: 10.1038/jcbfm.2014.111
Figures