0207
Simultaneous hemodynamic and structural imaging of ischemic stroke with MR Fingerprinting ASL1Department of Biomedical Engineering, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2The Russell H. Morgan Department of Radiology & Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
Synopsis
Tissue perfusion and structural imaging provides important information in the evaluation of ischemic stroke. MR-fingerprinting (MRF) arterial spin labeling (ASL) is a novel noninvasive method of ASL perfusion that allows simultaneous estimation of cerebral blood flow (CBF), bolus arrival time (BAT), and tissue T1 map in a single scan of <4 minutes. Here we evaluated the utility of MRF-ASL in ischemic stroke patients in depicting hemodynamic and structural abnormalities, as well as predicting neurological deficits and disability.
INTRODUCTION
MRF-ASL is a recently developed technique that allows simultaneous estimation of CBF, BAT, and tissue T1 in a single scan of < 4 minutes1,2. With the use of deep-learning reconstruction methods3-5, it has demonstrated reliable mapping of hemodynamic parameters. The present work aims to examine the utility of MRF-ASL in the assessment of ischemic stroke. We evaluated MRF-ASL parameters (i.e. CBF, BAT and T1) in brain regions corresponding to stroke, perilesional tissues, and contralateral normal brain tissues. We also examined the relationship between imaging parameters and the National Institutes of Health Stroke Scale (NIHSS) as well as the modified Rankin Scale (mRS). Finally, we determined the sensitivity of MRF-ASL parameters in differentiating stroke versus normal voxels in the brain.METHODS
MR experiments:34 ischemic stroke patients (57.97±13.70 yrs, 14 female) were scanned on a 3T Philips system. The imaging parameters of MRF-ASL: matrix size=64×64×7; voxel size=2.8×2.8×10 mm3; slice gap=1 mm; field-of-view (FoV)=180×180×76 mm3; TE=9.3ms; flip angle 40°; in-plane SENSE factor 2.4; multi-slice EPI; 500 time points; scan duration=3 min 45 sec. A T2-wighted image was acquired with the following parameters: matrix size=256×256×70; voxel size=0.8×0.8×2.2 mm3; FoV=212×212×154 mm3; TR=4200ms; TE=12ms; scan duration=3 min 28 sec. A diffusion tensor imaging (DTI) sequence was performed with the following parameters: matrix size=256×256×70; voxel size=0.8×0.8×2.2 mm3; FoV=212×212×154 mm3; TR=7000ms; TE=71ms; thirty-three gradient orientations with b=0 and 700 s/mm2; scan duration=4 min 34 sec.
Data processing:
Details of the MRF-ASL processing have been described previously5. Briefly, we used an artificial-neural-network (ANN) to reconstruct parametric maps from the raw image series. A total of four parametric maps were obtained from the MRF-ASL scan: CBF1-compartment, CBF2-compartment, BAT, and T1. Structural images were co-registered to the MRF-ASL space while preserving in-plane resolution. To quantify MRF-ASL parameters in different brain regions, regions-of-interest (ROIs) of ischemic lesion and contralateral normal tissues were manually delineated (Figure 1) by an imaging scientist based on co-registered structural images. A board-certified neuroradiologist also reviewed, adjusted, and confirmed these manually delineated ROIs. DWI was used for ROI drawing in stroke patients within 2 weeks after onset of symptoms (N=10). T2-weight images were used in patients >=2 weeks after onset (N=24). We also dilated the ischemic lesion ROI by 3.2 mm and referred to these dilated voxels as “perilesional tissue ROI”.
Statistical analysis:
Wilcoxon signed-rank tests were performed to compare MRF-ASL parameters between lesion, perilesional, and normal ROIs. Regression analysis was performed to evaluate the association between NIHSS (or mRS) and MRF-ASL parameters in lesion relative to normal region (i.e. Parameterlesion−Parameternormal). A p value (with Bonferroni correction when applicable) of 0.05 or less was considered significant. Receiver-operating-characteristic (ROC) curves were applied to the voxel-wise data to study the sensitivity and specificity of MRF-ASL parameters in correctly classifying voxels within the lesion and normal ROIs.
RESULTS AND DISCUSSION
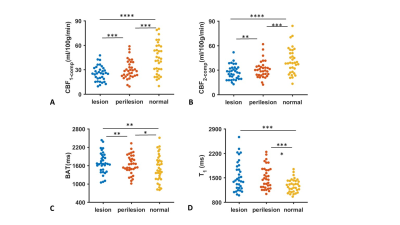
MRF-ASL parametric maps of four representative stroke patients with a range of NIHSS values are shown in Figure 1. Lesion and contralateral normal ROIs are also displayed.Figure 2 summarizes MRF-ASL parametric values in ROIs corresponding to stroke lesions, perilesional, and contralateral normal ROIs for CBF1-compartment, CBF2-compartment, BAT, and T1. Wilcoxon signed-rank tests revealed a significant difference in MRF-ASL parameters between lesion and normal ROIs in all four parameters (p<0.01). Specifically, stroke lesions manifested lower CBF1-compartment, lower CBF2-compartment, longer BAT and longer T1 compared to normal ROIs. Perilesional parametric values were generally in-between lesion and normal ROI values (p<0.05), except for T1, where there was no significant difference between perilesional and lesion values.
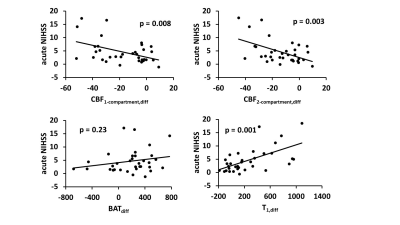
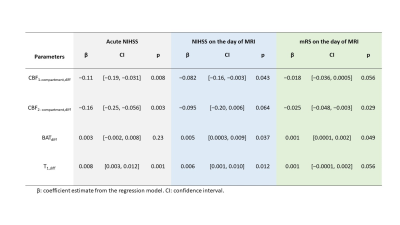
Table 1 summarizes the associations between MRF-ASL parameters and NIHSS and mRS scores. We found a negative association between acute NIHSS score and CBF1-compartment,diff (p=0.008) and between acute NIHSS score and CBF2-compartment,diff (p=0.003), and a positive association between acute NIHSS score and T1,diff (p=0.001). That is, a lower CBF1-compartment, lower CBF2-compartment, and longer T1 are associated with greater neurological deficits at the acute stage. BATdiff did not show an association with the acute NIHSS score. Figure 3 shows a scatter plot between MRF-ASL parameters and acute NIHSS score. Similar relationships were observed (Table 1) when comparing MRF-ASL parameters with NIHSS and mRS scores acquired on the day of MRI (i.e. non-acute scores). However, the associations with the non-acute scores were generally weaker.
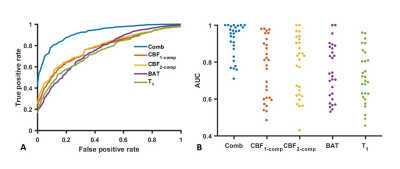
Figure 4A shows group-averaged ROC curves of using MRF-derived CBF1-compartment, CBF2-compartment, BAT, T1, and their combination in the classification of lesion versus normal voxels. Figure 4B displays the AUC of ROC curves corresponding to these classification schemes. The combination of all four MRF-ASL parameters yielded the highest AUC (0.92±0.09). The other AUC values were: 0.77±0.17 for CBF1-compartment alone, 0.78±0.17 for CBF2-compartment alone, 0.75±0.14 for BAT alone, and 0.73±0.14 for T1 alone. AUC value of ROC curve using the combination of MRF-ASL parameters for classification is considerably higher than any of the single parameter alone (p<0.001). No significant differences in AUC values were observed between the single-parameter results. It was also noted that different patients revealed different sensitivities in parametric maps.
CONCLUSIONS
Novel MRF-ASL technique allows simultaneous mapping of CBF, BAT, and tissue T1 deficits in ischemic stroke. MRF-ASL derived parameters were predictive of NIHSS and mRS scores and capable of classifying lesion voxels with an accuracy of 92%.Acknowledgements
No acknowledgement found.References
1. Su P, Mao D, Liu P, Li Y, Pinho MC, Welch BG, Lu H. Multiparametric estimation of brain hemodynamics with MR fingerprinting ASL. Magn Reson Med 2017;78:1812-1823.
2. Wright KL, Jiang Y, Ma D, Noll DC, Griswold MA, Gulani V, Hernandez-Garcia L. Estimation of perfusion properties with MR Fingerprinting Arterial Spin Labeling. Magnetic Resonance Imaging 2018;50:68-77.
3. Lahiri A, Fessler JA, Hernandez-Garcia L. Optimizing MRF-ASL scan design for precise quantification of brain hemodynamics using neural network regression. Magn Reson Med 2020;83:1979-1991.
4. Zhang Q, Su P, Chen ZS et al. Deep learning-based MR fingerprinting ASL ReconStruction (DeepMARS). Magnet Reson Med 2020;84:1024-1034.
5. Fan HL, Su P, Huang J, Liu PY, Lu HZ. Multi-band MR fingerprinting (MRF) ASL imaging using artificial-neural-network trained with high-fidelity experimental data. Magnet Reson Med 2021;85:1974-1985.
Figures