0187
Twenty-channel, Highly-stretchable, Overlapped, Receive (THOR) Array
Jana M. Vincent1,2,3,4, Fraser Robb4, Victor Taracila4, and Joseph V. Rispoli1,2
1Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 2School of Electrical and Computer Engineering, Purdue University, West Lafayette, IN, United States, 3Department of Basic Medical Sciences, Purdue University, West Lafayette, IN, United States, 4MR Engineering, GE Healthcare Coils, Aurora, OH, United States
1Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 2School of Electrical and Computer Engineering, Purdue University, West Lafayette, IN, United States, 3Department of Basic Medical Sciences, Purdue University, West Lafayette, IN, United States, 4MR Engineering, GE Healthcare Coils, Aurora, OH, United States
Synopsis
There has been a trend towards lightweight, flexible coils that provide greater SNR and patient comfort. To improve upon these coils and expand on existing stretchable designs, we present an omnidirectionally-stretchable, twenty-channel coil made with conductive thread on athletic fabric for use as a multipurpose coil. This coil was used to obtain in vivo images of anatomies including the wrist and ankle. Greater image quality and sensitivity can be seen with the stretchable coil compared to the commercial flexible coil. The stretchable coil was easier to place, able to be positioned more closely, and was more comfortable for the subjects.
Introduction
With the development of ultra-flexible coil arrays1,2, there has been an increase in both conformability and signal-to-noise ratio (SNR) as well as enhanced patient comfort3,4. One area of development has been in the creation of stretchable coil arrays, which provide superior contouring around curved surfaces and greater availability of coil placement across a variety of anatomies and body sizes5-18. These stretchable coils provide greater adaptability than previously seen with other commercial coils. In joint applications, coils with a greater degree of conformability and stretch allow for imaging at different degrees of flexion which, in turn, provide enhanced comfort for those unable to withstand traditional positioning, whether it be due to injury or disease. Compared to existing stretchable coil designs, the presented coil has a higher channel count with small, 7-cm elements.Methods
Fabrication of the 20-channel, 7-cm loop, stitched array was completed using silver-coated p-phenylene benzobisoxazole (PBO) thread (Lyofil-332, Syscom Advanced Materials Inc., Columbus, OH, USA) on a stretch knit fabric. Prior to the array embroidery, Q-measurements for a single stitched loop stretched in various configurations within a plastic embroidery hoop were made using a set of decoupled pickup probes for S21 measurements with the loop loaded and unloaded. Utilizing a professional embroidery machine, the 20 loops were stitched on the fabric and anchored by polyester thread in a 5 x 4 element arrangement. Each loop was segmented with a tuning capacitor and an AIR™ coil module (GE Healthcare, Waukesha, WI, USA) comprised of a match/tune circuit, balun, and a custom preamplifier placed adjacent to the tuning capacitor on each loop (Fig.1). The coil was covered in a biocompatible sleeve for cleanability (Fig. 1). Prior to in vivo scanning, coil SNR was calculated from a phantom scan in relaxed and stretched positions using a 3T scanner (SIGNA Premier, GE Healthcare, Waukesha, WI, USA) and by obtaining signal and pure noise scans. In vivo scans were performed on a different 3T scanner (MR750, GE Healthcare, Waukesha, WI, USA) across individuals of varying body sizes. Preset scan protocols for the ankle and wrist were used and all scanning parameters were kept the same between the stretchable coil and the commercial, 16-channel flexible coil of comparable size (Medium GEM Flex Coil, NeoCoil, Pewaukee, WI, USA). The full scan protocol and parameters for the presented image comparisons are summarized in the corresponding subfigures. The experimental procedures described in this abstract were approved by the local Institutional Review Board (protocol 19030219).Results
The preamplifier decoupling was greater than 850 Ω, the noise figure was less than 0.5 dB, and the gain was 37 dB. The Q-ratio of a single stitched loop was 2.07 in the relaxed position, 2.12 in the horizontally stretched position, 2.55 in the vertically stretched position, and 1.43 when stretched omnidirectionally. SNR measurements are summarized in Fig. 2. An in vivo ankle scan image for both the stretch and commercial flex coil are shown in Fig. 3 along with the scan parameters. The comparison of images obtained for the wrist with both coils and the scan parameters are shown in Fig. 4.Discussion
A high blocking impedance was needed to account for coupling losses given the variability in loop size and conformation. For all stretch configurations, except omnidirectional, the coil loop had a Q-ratio over 2 which corresponds to body/sample-noise dominance. Overall, no degradation in SNR was seen after the coil was stretched and relaxed during phantom and in vivo scanning. SNR values were similar across different stretch configurations; however, the omnidirectionally-stretched coil maintained slightly higher SNR at greater phantom depths, which was expected due to the larger element size. In the in vivo scans, the stretchable coil provided increased definition of the ankle joint and tissue. This is due to the closer placement allowed around the joint compared to the more rigid/less contourable flex coil. Fig. 1c highlights the close fit of the stretchable coil around the ankle. The stretchable nature also allowed for the ankle to be freely wrapped and imaged at other bend angles besides the 90° positioning typically seen in clinical scans. The wrist scans also exhibited increased definition and more uniform signal around the surface due to the closer contour and smaller element size of the stretchable coil.Conclusion
At the time of this abstract submission, this is the highest channel count, fully-stretchable array. This coil exhibited improvement in patient positioning and comfort due to the soft, flexible, and stretchable design. With this coil, joints are not constrained to certain positions allowing for imaging at different degrees of flexion. The high blocking impedance from the novel preamplifier greatly facilitated the change in loop size and overlap when stretched. Compared to a commercial flex coil of similar size, the stretchable coil demonstrated improved definition of the joint and tissue as well as a more uniform signal distribution. Overall, this coil array can be used for a variety of anatomies and body sizes. Further research into different conductive threads to reduce the resistance and improve coil performance is a high priority. Additional research on loop diameters and increasing channel counts are also of interest to create varying sizes of multipurpose arrays.Acknowledgements
The authors would like to thank Kristine Barnhart for her expertise and assistance with scan protocol development and Antonia Susnjar for all of her efforts in coordinating and assisting with scans.References
- Rossman P., et al., “Characterization of a new ultra-flexible low-profile RF receive coil technology,” Proc Intl Soc Mag Reson Med, 2017, vol 25, 0763.
- Vasanawala S.S., et al., “Development and Clinical implementation of very light weight and highly flexible Air technology arrays,” Proc Intl Soc Mag Reson Med, 2017, vol. 25, 0755.
- Vincent J., et al., “Ultra-Flexible, High-Resolution, 60-Channel RF Coil for Supine Breast Imaging,” Proc Intl Soc Mag Reson Med, 2021, vol. 29, 1591.
- Stickle Y-J., et al., “A Novel Ultra-Flexible High-Resolution AIR (Adaptive Imaging Receive) 64-Channel Bilateral Phased Array for 3T Brachial Plexus MRI,” Proc Intl Soc Mag Reson Med, 2020, vol. 28, 4025.
- Vincent, J. and Rispoli, J., "Novel Stretchable and Flexible Radiofrequency Coil Design for Magnetic Resonance Imaging," in BMES Annual Meeting, Phoenix, AZ, October 11-14 2017, pp. P-FRI-329.
- Vincent, J.M. and Rispoli, J.V., "Stitching Stretchable Radiofrequency Coils for MRI: A Conductive Thread and Athletic Fabric Approach," 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 23-27 July 2019 2019, pp. 6798-6801, doi: 10.1109/EMBC.2019.8857051.
- Vincent, J.M. and Rispoli, J.V., "Two-Channel, Stretchable, and Flexible Breast Coil Array," in Proc Intl Soc Mag Reson Med. 28, Virtual, 2020, 4275.
- Varga, M., et al., "Adsorbed Eutectic GaIn Structures on a Neoprene Foam for Stretchable MRI Coils," Adv Mater, vol. 29, no. 44, Nov 2017, doi: 10.1002/adma.201703744.
- Mehmann, A., et al., "On the Bending and Stretching of Liquid Metal Receive Coils for Magnetic Resonance Imaging," IEEE Trans Biomed Eng, vol. 66, no. 6, pp. 1542-1548, Jun 2019, doi: 10.1109/TBME.2018.2875436.
- Port, A., et al., "Liquid metal in stretchable tubes: A wearable 4-channel knee array," Proc Intl Soc Mag Reson Med, Montreal, QC, Canada, 2019, vol. 27, 1114.
- Port, A., Luechinger, R., Brunner, D.O., and Pruessmann, K.P., "Elastomer coils for wearable MR detection," Magnetic Resonance in Medicine, vol. 85, no. 5, pp. 2882-2891, 2021, doi: https://doi.org/10.1002/mrm.28662.
- Port, A., et al., "Towards wearable MR detection: A stretchable wrist array with on-body digitization," in Proc Intl Soc Mag Reson Med, Paris, France, 2018, vol. 26, 0017.
- Nordmeyer-Massner, J.A., et al., "MR imaging of healthy knees in varying degrees of flexion using a stretchable coil array provides comparable image quality compared to a standard knee coil array," European Journal of Radiology, vol. 85, pp. 518-523, 2016, doi: 10.1016/j.ejrad.2015.12.004.
- Agir, B.K., et al., "A Wearable and Flexible 23Na Transmit/Receive Breast Coil at 3T," Proc Intl Soc Mag Reson Med 28, 2020, #4104.
- Port, A., et al., "Detector clothes for MRI: A wearable array receiver based on liquid metal in elastic tubes," Scientific Reports, vol. 10, no. 1, p. 8844, 2020/06/01 2020, doi: 10.1038/s41598-020-65634-5.
- Kahraman-Agir, B., Yegin, K., and Ozturk-Isik, E., "Wearable and Elastic Surface Coil for 1H Magnetic Resonance Imaging," IEEE Microwave and Wireless Components Letters, pp. 1-1, 2021, doi: 10.1109/LMWC.2021.3068930.
- Motovilova, E., et al., “Self-tuning stretchable RF receive coil concept using liquid metal encapsulated within an elastic polymer,” Proc Intl Soc Mag Reson Med, Virtual, 2020, vol. 28, 0136.
- Motovilova, E., et al., “Stretchable self-tuning MRI receive coils based on liquid metal technology (LiquiTune),” Sci Rep 11, 16228 (2021). https://doi.org/10.1038/s41598-021-95335-6.
Figures
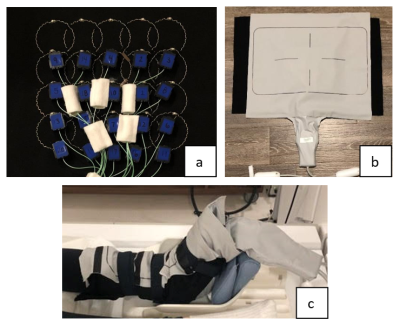
Figure 1. a) Internal
assembly, b) Complete assembly, c) Example of positioning on an ankle.
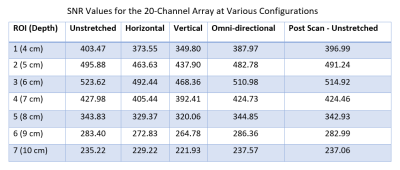
Figure 2. Summary of the change in SNR for various stretch configurations at increasing phantom
depths.
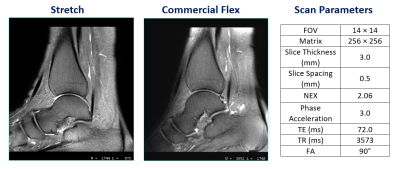
Figure 3. Sagittal T2
fast spin echo (FSE) fat saturated (FS) propeller ankle scan from the stretch
coil (left) and flex coil (center). The scan parameters for this scan are
summarized on the right.
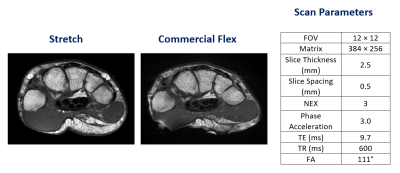
Figure 4. Axial T1-weighted FSE scan of
the wrist with the stretch coil (left) and flex coil (center) with the corresponding
scan parameters in the table on the right.
DOI: https://doi.org/10.58530/2022/0187