0184
Hierarchical multi-resolution graph-cuts for water–fat–silicone separation in breast MRI1Department of Diagnostic and Interventional Radiology, Technical University of Munich, Munich, Germany, 2Department of Physics, Technical University of Munich, Garching, Germany, 3Philips Healthcare, Hamburg, Germany
Synopsis
A water- and fat-suppressed sequence is typically used to verify the integrity of silicone breast implants. The feasibility of using chemical shift encoding-based MRI for in vivo water–fat–silicone separation was recently shown. In this work, a hierarchical multi-resolution graph-cut framework is developed and proposed for water–fat–silicone separation in breast MRI. Robustness and competitive processing times are achieved by performing graph-cuts using varying spatial resolution. Results show successful water–fat–silicone separation for 4 echoes (the minimum of required echoes) without water–fat–silicone swaps and an improved silicone contrast compared to the conventional sequence.
Purpose
Multi-echo gradient echo imaging acquisitions have been recently combined with chemical shift encoding-based water–fat separation (CSE-WFS) for quantifying water–fat composition1,2 and for extracting magnetic susceptibility maps3-5 in breast MRI. It has also been shown that CSE-WFS can be utilized for the robust separation of additional components such as silicone6. Silicone-separated images might allow the replacement of water- and fat-suppressed silicone-only sequences to detect implant’s ruptures in breast MRI7,8, at an improved resolution and signal-to-noise ratio (SNR).A previously introduced method based on a single graph-cut needs however a minimum of 6 echoes for the robust separation of water, fat and silicone and the parameters might need to be adjusted for different subjects6. In this work, a hierarchical multi-resolution graph-cut framework consisting of multiple graph-cut layers is presented for the joint separation of water and fat or water, fat and silicone. Each layer allows for different signal models, spatial resolution, regularization, and sampling ranges enabling a fast and more robust chemical species separation.
Methods
Proposed method (hmrGC-wfs)The water–fat voxel signal model was extended for silicone and its chemical shift6. A variable projection method was applied to solve firstly for the field-map9. For each layer in the presented framework, residuals were calculated, and all local minima were extracted and inserted to a single-min-cut variable-layer graph-cut (vlGC) algorithm10. In difference to the previously introduced method for water–fat–silicone separation6 (vlGC-wfs) several modifications were applied:
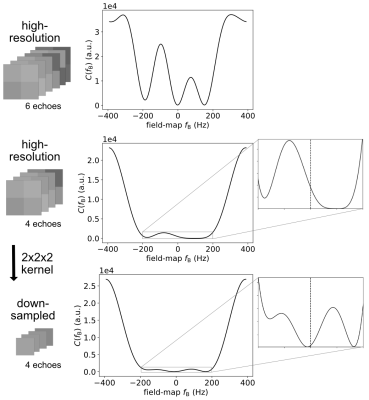
Spatial resolution: The 3D-image array can be downsampled to decrease the number of graph nodes and reduce sensitivity to noise of the residuals (Fig. 1).
Signal model and regularization parameter: The graph-cut algorithm can be performed for different signal models (water–fat or water–fat–silicone) and regularization parameters.
Field-map priors: Prior field-maps can be used as an initialization for the graph-cut algorithm by setting the sampling neighborhood or including the prior as graph nodes in the graph-cut algorithm.
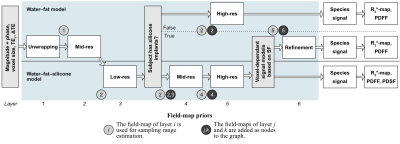
The proposed hierarchical multi-resolution method (hmrGC-wfs) consists of multiple connected graph-cut layers with different properties summarized in Fig. 2. First, the unwrapped field-map range is found in a low-resolution layer. Subsequently, the resolution is increased, and it is determined based on the silicone fraction if silicone implants are present. If no silicone implants are present, a water–fat model is applied, and a high-resolution field-map is computed. Otherwise, the water–fat and water–fat–silicone model is chosen voxel-wise based on the silicone fraction and a high-resolution field-map is computed similarly. The presence of silicone implants can also be a prior information. Species signals, $$$R_2^*$$$-maps and the proton-density fat fraction (PDFF) are calculated afterwards based on the field-map11-13. The proton-density silicone fraction (PDSF) is a relative estimate of silicone per voxel and is calculated similar to the PDFF with correction for noise bias effects.
MR measurements
MR measurements were performed in 20 subjects receiving breast examinations in a 3T scanner (Philips Ingenia, Philips Healthcare, Best, The Netherlands). 10 subjects have silicone implants with 5 subjects with bilateral silicone implants and 5 subjects with an unilateral silicone implant. A monopolar time-interleaved multi-echo gradient echo sequence14 was used with 6 echoes, TE1=1.58 ms, ∆TE=1.28 ms, flip angle: 3°, TR=12.21 ms, FOV=220x382.6x192.4 mm³, 1.3 mm isotropic resolution, C-SENSE with R=6 and scan time of 4:15 min. Either all 6 or only the first 4 echoes were used for water–fat–silicone separation. Method hyperparameters (e.g., voxel sizes for downsampled images, regularization parameter) were manually adjusted based on a smaller validation dataset with similar scan parameters. Results were compared with a clinical silicone-only scan with water and fat suppression (T2-weighted turbo spin echo DIR acquisition, TE=65 ms, TI1/TI2=3100/220 ms, TR=15.7 s, C-SENSE with R=3.5, acquired voxel size: 1.25x1.5x3 mm³, FOV=220x382.6x192.4 mm³, scan time: 3:08 min).
Performance evaluation
The performance of the proposed algorithm was compared with the previous presented vlGC algorithm for water–fat separation10 and its modification for water–fat–silicone separation6 (vlGC-wfs). Both methods use a field-map sampling range of $$$2.5*f_{\text{B,period}}$$$ with the period length $$$f_{\text{B,period}}=\frac{1}{\Delta \text{TE}}$$$ to allow an unwrapped field-map estimate.
Results
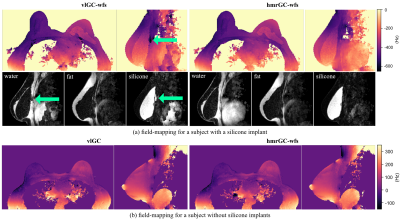
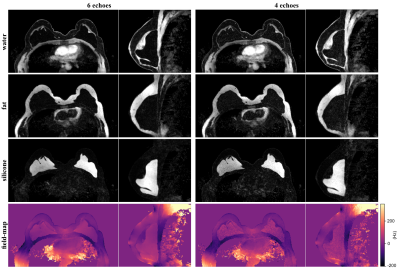
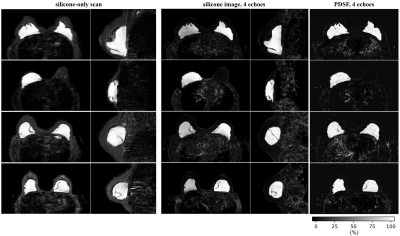
The comparison with the vlGC and vlGC-wfs algorithms shows a similar field-map accuracy for the hmrGC-wfs algorithm if the subject does not have silicone breast implants (Fig. 3). For a subject with silicone implants and 4 echoes, water–fat–silicone swaps are occurring in the vlGC-wfs algorithm. The hmrGC-wfs algorithm minimizes chemical species swaps and significantly decreases processing times (decrease of 85% in subjects with silicone implants, and a decrease of 95% in subjects without implants compared to the vlGC-wfs/vlGC algorithm). Similar separation performance is achieved for 4 and 6 echoes with a slightly degraded noise performance for 4 echoes (Fig. 4). Water-separated images enabled the delineation of the fibrous capsule surrounding the implant (Fig. 4). Silicone-separated images showed a higher spatial resolution and an improved contrast with better fat suppression compared to the conventional silicone-only scan (Fig. 5).Discussion & Conclusion
A hierarchical multi-resolution graph-cut framework was developed and proposed for water–fat–silicone separation in breast MRI. Robustness and competitive processing times are achieved by performing graph-cuts using varying spatial resolution. The hierarchical use of signal models and the linkage of graph-cut layers assure a high field-map accuracy, without water–fat–silicone swaps. The derived silicone-separated images might have the ability to overcome limitations of clinically used silicone-only scans.Acknowledgements
This work was supported in part by the European Research Council (grant agreement No 677661, ProFatMRI). The authors also acknowledge research support from Philips Healthcare.References
1. Ding J, Stopeck AT, Gao Y, et al. Reproducible automated breast density measure with no ionizing radiation using fat-water decomposition MRI. J Magn Reson Imaging. 2018;48(4):971-981. doi:10.1002/jmri.26041.
2. Borde T, Wu M, Ruschke S, et al. Assessing breast density using the standardized proton density fat fraction based on chemical shift encoding-based water-fat separation. Proc. Intl. Soc. Mag. Reson. Med. 29. 2021:743.
3. Dimov AV, Liu T, Spincemaille P, et al. Joint estimation of chemical shift and quantitative susceptibility mapping (chemical QSM). Magn Reson Med. 2015;73(6):2100-2110. doi:10.1002/mrm.25328.
4. Fatemi-Ardekani A, Boylan C, Noseworthy MD. Identification of breast calcification using magnetic resonance imaging. Med Phys. 2009;36(12):5429-5436. doi:10.1118/1.3250860.
5. Schweser F, Herrmann K-H, Deistung A, et al. Quantitative magnetic susceptibility mapping (QSM) in breast disease reveals additional information for MR-based characterization of carcinoma and calcification. Proc. Intl. Soc. Mag. Reson. Med. 19. 2011:1014.
6. Stelter JK, Boehm C, Ruschke S, et al. Optimal experimental design for quantitative water, fat and silicone separation using a variable projection method with 4 or 6 echoes at 3T. Proc. Intl. Soc. Mag. Reson. Med. 29. 2021:3848.
7. Mukundan S, Dixon WT, Kruse BD, Monticciolo DL, Nelson RC. MR imaging of silicone gel-filled breast implants in vivo with a method that visualizes silicone selectively. J Magn Reson Imaging. 1993;3(5):713-717. doi:10.1002/jmri.1880030505.
8. Monticciolo DL, Nelson RC, Dixon WT, Bostwick J, Mukundan S, Hester TR. MR detection of leakage from silicone breast implants: value of a silicone-selective pulse sequence. AJR Am J Roentgenol. 1994;163(1):51-56. doi:10.2214/ajr.163.1.8010247.
9. Hernando D, Haldar JP, Sutton BP, Ma J, Kellman P, Liang Z-P. Joint estimation of water/fat images and field inhomogeneity map. Magn Reson Med. 2008;59(3):571-580. doi:10.1002/mrm.21522.
10. Boehm C, Diefenbach MN, Makowski MR, Karampinos DC. Improved body quantitative susceptibility mapping by using a variable-layer single-min-cut graph-cut for field-mapping. Magn Reson Med. 2021;85(3):1697-1712. doi:10.1002/mrm.28515.
11. Yu H, McKenzie CA, Shimakawa A, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging. 2007;26(4):1153-1161. doi:10.1002/jmri.21090.
12. Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36(5):1011-1014. doi:10.1002/jmri.23741.
13. Liu C-Y, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58(2):354-364. doi:10.1002/mrm.21301.
14. Ruschke S, Eggers H, Kooijman H, et al. Correction of phase errors in quantitative water-fat imaging using a monopolar time-interleaved multi-echo gradient echo sequence. Magn Reson Med. 2017;78(3):984-996. doi:10.1002/mrm.26485.
Figures