0177
Rapid 3D Quantitative Mapping of Brain Metastases with Deep Learning-Based Phase-Sensitive MR fingerprinting1Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Radiation Oncology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
In MR fingerprinting, quantitative maps are obtained by matching the measured signal to a pre-computed dictionary. However, a key constraint of dictionary matching is the exponential growth of the dictionary with the number of parameters. A deep learning method named DRONE overcomes this constraint by using deep learning to map the magnitude-valued signal to the underlying tissue parameters. Here we describe an extension of DRONE that jointly estimates a phase term to enable mapping complex-valued signals and improve the quantitative accuracy. We test the accuracy in the ISMRM NIST phantom and demonstrate the clinical utility in patients with brain metastases.
Introduction
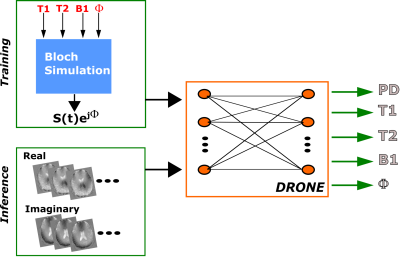
In MR fingerprinting (MRF), quantitative maps are obtained by dynamically varying the acquisition parameters and matching the resulting signal to a pre-computed dictionary[1]. Dictionary matching is constrained, however, by the exponential growth of the dictionary with the number of parameters. A recently described deep learning method named DRONE[2] overcomes this constraint by using a fully-connected neural network to map the magnitude signal to the underlying tissue parameters. In its original implementation, DRONE used a real-valued neural network to quantify T1 and T2 from magnitude images. But relaxometry from magnitude images is susceptible to errors due to, for example, ambiguities in the zero-crossing of the signal or the non-zero noise mean[3]. A simple splitting of the image into real and imaginary components blurs the relationship between the components and can also lead to erroneous results. Some groups have proposed the use of complex networks[4] but complex operations and optimization algorithms are poorly supported by current deep learning software frameworks. Instead, this work proposes a straightforward modification of DRONE that enables reconstruction of complex data using real-valued networks and also yields potentially useful phase maps. The proposed method is called ‘Phase-Sensitive DRONE’ (PS-DRONE). The accuracy of our method is characterized in the ISMRM NIST phantom[5] and the clinical utility demonstrated in a healthy human subject and patients with brain metastases.Methods
Pulse sequenceImages were acquired with an EPI based MRF pulse sequence (MRF-EPI) whose 50-point schedule of flip angles (FA) and repetition times (TR) was optimized to maximize tissue discrimination[6]. The remaining acquisition parameters were as follows: partial Fourier factor of ~6/8, acceleration factor R=3, echo time=24 ms, matrix size=224×224, FOV=280 mm2 for an in-plane resolution of 1.25 mm2 and a slice thickness of 5 mm. The scan time was 5 seconds per slice.
PS-DRONE
The outline of the PS-DRONE method is shown in Figure 1. Like its predecessor, PS-DRONE uses a training dataset of signal magnetizations generated by simulating an MRF acquisition for different tissue parameter values. In PS-DRONE, however, each signal includes a multiplicative phase term exp(jΦ) that accounts for phase variations in the signal. In the network inference stage, the value of Φ for each voxel is estimated along with the other parameters (i.e. T1, T2 and B1) In this work, the network was trained with a 400,000 entries dictionary selected from the following ranges: T1=[1, 4000], T2=[1, 3000], B1=[0, 1.5], Φ=[-π, π]. The proton density (PD) was calculated as a scaling factor from the reconstructed data.
Phantom experiments
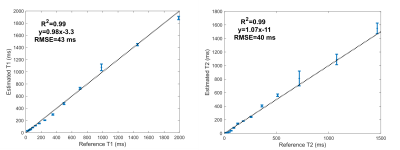
All experiments were conducted on a Signa Premier 3T scanner (GE Healthcare, Waukesha, WI) with a 48-channel head receiver coil. The ISMRM NIST phantom was scanned with the MRF-EPI sequence and the T1 and T2 maps quantified with PS-DRONE. Regions-of-interest were drawn around each compartment and the mean and standard deviation calculated and compared to the reference values.
In vivo healthy subject
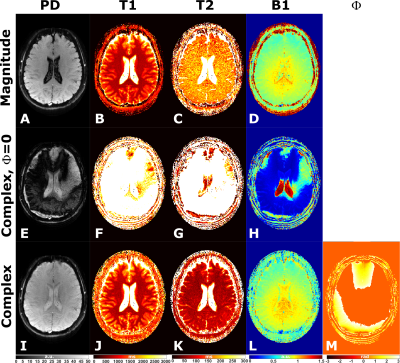
A healthy, 30 years old female volunteer was recruited and gave informed consent in accordance with the institutional IRB protocol. The subject was scanned with the MRF-EPI sequence and the data reconstructed with PS-DRONE as described above. For comparison, we also reconstructed the data using conventional DRONE using the magnitude images and the complex data but without the phase estimation.
Brain metastases subjects
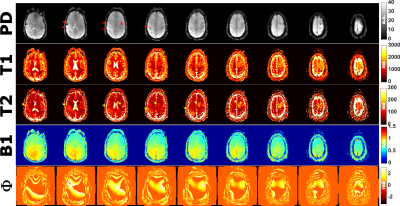
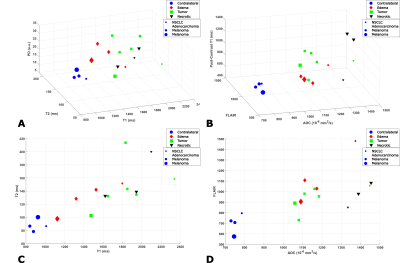
Three subjects with metastatic brain tumors were recruited for this study and gave informed consent. The tissue maps were quantified with PS-DRONE and each lesion segmented into tumor, necrotic and edema regions by a trained radiation oncologist. A healthy contra-lateral region was also demarcated. The mean and standard deviation of the tissue parameter values in each region were compared across patients and compared to values obtained with a standard-of-care protocol in the same scan session for each patient.
Results
T1 and T2 values in the phantom reconstructed with PS-DRONE presented strong agreement (R2=0.99) with the reference values (Figure 2). The reconstruction of the in vivo data for the healthy subject is shown in Figure 3. Reconstructions using the magnitude (Fig. 3A-D) and the complex data without phase estimation (Fig. 3E-H) showed significant artifacts or quantification errors, unlike the phase estimated complex reconstruction (Fig. 3I-M). An example of tissue maps in a subject with metastatic melanoma is shown in Figure 4. A comparison between the quantitative MRF maps and standard-of-care protocols across the lesions is shown in Figure 5.Discussion/Conclusion
This work demonstrated a novel phase-sensitive deep learning quantification of MRF data. Phase mapping is an integral part of multiple pulse sequences[7]. The phase maps obtained with PS-DRONE may similarly yield clinically valuable data after appropriate processing, but this is left for future studies. Metastatic brain lesions are frequently multi-focal so whole brain coverage is necessary. Unfortunately, standard-of-care protocols are already lengthy so minimizing scan time is important. In combination with the optimized MRF-EPI pulse sequence, our approach enables whole head mapping of multiple parameters in less than 5 minutes and can be easily added to existing protocols.Acknowledgements
This work was partially supported by the NIH/NCI Cancer Center Support Grant/Core Grant (P30 CA008748).References
[1] D. Ma et al., “Magnetic resonance fingerprinting,” Nature, vol. 495, no. 7440, pp. 187–192, 2013.
[2] O. Cohen, B. Zhu, and M. S. Rosen, “MR fingerprinting deep reconstruction network (DRONE),” Magn. Reson. Med., vol. 80, no. 3, pp. 885–894, 2018.
[3] N. Stikov, M. Boudreau, I. R. Levesque, C. L. Tardif, J. K. Barral, and G. B. Pike, “On the accuracy of T1 mapping: searching for common ground,” Magn. Reson. Med., vol. 73, no. 2, pp. 514–522, 2015.
[4] P. Virtue, X. Y. Stella, and M. Lustig, “Better than real: Complex-valued neural nets for MRI fingerprinting,” in 2017 IEEE international conference on image processing (ICIP), 2017, pp. 3953–3957.
[5] K. F. Stupic et al., “A standard system phantom for magnetic resonance imaging,” Magn. Reson. Med., 2021.
[6] O. Cohen and M. S. Rosen, “Algorithm comparison for schedule optimization in MR fingerprinting,” Magn. Reson. Imaging, vol. 41, pp. 15–21, 2017.
[7] Y. Wang and T. Liu, “Quantitative susceptibility mapping (QSM): decoding MRI data for a tissue magnetic biomarker,” Magn. Reson. Med., vol. 73, no. 1, pp. 82–101, 2015.
Figures