0170
Triplanar Ensemble Detection Network (TPE-Det): A Single End-to-End Model for Efficient Detection of Cerebral Microbleeds in MR Images1Department of Electrical & Electronic Engineering, Yonsei University, Seoul, Korea, Republic of, 2Neuroscience Research Institute, Gachon University, Incheon, Korea, Republic of, 3Department of Neurology, Gachon University College of Medicine, Gil Medical Center, Incheon, Korea, Republic of
Synopsis
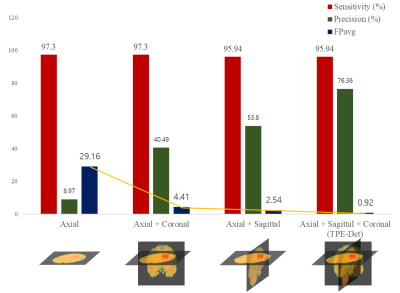
Automated detection of Cerebral Microbleeds (CMBs) in Magnetic Resonance (MR) images can be clinically useful because of the small size of CMBs and the presence of numerous mimics. In this study, we propose an end-to-end Triplanar Ensemble Detection Network (TPE-Det) that demonstrates notable performance with only a single stage. Via the proposed TPE-Det, we combined CMBs detection results from axial, sagittal, and coronal planes. When considering the additional planes, the average False Positives per patient (FPavg) decreased from 29.16 to 0.92 compared to the case of using only the axial plane, preserving the high sensitivity of 95.94%.
Introduction
CMBs are microscopic cerebral hemorrhages. They can be clinical implications for various diseases such as cerebrovascular disease, ischemic stroke, and Alzheimer's disease1. Therefore, for proper diagnosis and treatment, it is important to accurately distinguish CMBs from their mimics such as calcifications, irons, and veins in brain MR images.However, automated detection of CMBs is a challenging task due to their wide distribution throughout the brain, small sizes, and visual similarity to mimics. For this reason, most of the previously proposed methods consist of two stages: candidate detection stage and False Positive (FP) reduction stage2-6. In this study, we propose a novel end-to-end TPE-Det that demonstrates improved performance with only a single stage utilizing the ensemble of 2D CNN-based networks.
Methods
[Dataset]Our data were acquired using 3.0 T Skyra Siemens MRI scanners. A total of 114 subjects including 365 microbleeds were collected with the following imaging parameters: the resolution of 0.50×0.50×2 mm3, repetition time (TR) of 27 ms, echo time (TE) of 20 ms, flip angle (FA) of 15◦, and field of view (FOV) of 256×224 mm2. In our experiments, we randomly divided the whole dataset into three portions: 85 patients with 274 CMBs for training, 6 patients with 17 CMBs for validation, and 23 patients with 74 CMBs for testing.
Susceptibility-weighted imaging (SWI) assists in improving the discrimination of CMBs from other mimics7. However, it is difficult to differentiate CMBs from calcifications only using the SWI images because both of them appear with similar shapes and intensities. To solve this problem, we utilized a combination of phase data along with the SWI image, since diamagnetic calcification can be separated from paramagnetic CMBs based on the sign of the phase3.
[Detection Networks]
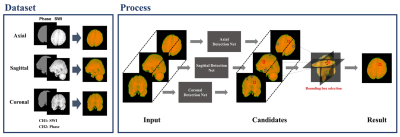
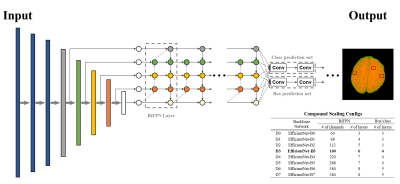
The whole detection procedure is summarized in Fig.1. Each detection network is based on EfficientDet8, which is one of the state-of-the-art 2D object detection networks. The architecture of the model is shown in Fig.2. The network can efficiently detect objects due to the Bi-directive Feature Pyramid Network (BiFPN) and compound scaling technique. The BiFPN is the feature network combining low-resolution, strongly semantic features with high-resolution, weakly semantic features which are crucial to detect CMBs with minuscule sizes. And the compound scaling is jointly scaling up the network width, depth, and input resolution to optimize the network’s size and improve efficiency. Since our ensemble approach requires three detection networks, we save computation cost by employing EfficientDet D3 from the compound scaling.
[Bounding Box Selection]
First, we independently detect the CMBs using axial, sagittal, and coronal images via the proposed TPE-Det. Then, the three-dimensional coordinate of each detection is calculated from its x, y coordinates, and slice number. Afterwards, we calculate the Euclidean distances between the candidates detected from each plane and deem the objects single if they are closer than a pre-set distance threshold value. Based on this method, the candidates that are detected on all three planes are considered as our final detections. As a result, we are left with only candidates which have morphological characteristics of CMBs in every plane without employing an additional stage.
Results
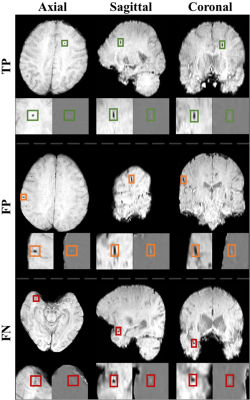
The sensitivity, precision, and FPavg were observed by various plane combinations (Fig.3). As considering more planes, the precision increased and FPavg decreased dramatically conserving high sensitivity. Fig.4 shows True Positive (TP), FP, and False Negative (FN) examples via the proposed TPE-Det. TP CMBs commonly had a small spherical shape in the axial plane but an elongated ellipse shape in the sagittal and coronal planes. FPs had similar intensities and shapes with CMBs. They were usually veins appearing with mimic shapes in a few slices. FNs were generally too small and vague CMBs to be detected. Besides, the FN in Fig.4 shows small spherical shapes in sagittal and coronal, different from normal CMBs.Discussion & Conclusion
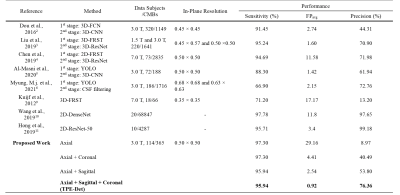
In this study, we suggest the novel TPE-Det, which showed notable improvements for CMBs detection by combining multi-plane detections as a single-stage end-to-end approach, despite using the dataset with anisotropic resolution. The experiment results showed the importance of 3D information in detecting CMBs. TPE-Det could utilize the three-dimensional information by simply taking CMBs detected in all three planes, without employing 3D networks. In addition, we present comparisons with the latest related studies (Fig.5). Note that since the utilized datasets among all previous methods are not the same, it does not show an exact quantitative comparison. As shown in Fig.5, the proposed TPE-Det detected CMBs with a notably small number of FPavg preserving high sensitivity.However, there are three main limitations to this study. First is the limited size of labeled data, which is a general problem in deep learning methods for medical image analysis. Second, our ensemble approach requires independent training of the proposed network three times for three different MRI planes. Lastly, the characteristics of CMBs could differ by disease and scan parameter, resulting in difficulty to develop a general model.
Because the FPs were usually veins, as shown in Fig.4, it will be valuable to perform vessel segmentation for FPs reduction in post-processing as further work.
Acknowledgements
This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2018M3C7A1056884).
References
1. Martinez-Ramirez S, Greenberg SM, Viswanathan A. Cerebral microbleeds: overview and implications in cognitive impairment. Alzheimers Res Ther 2014;6(3).
2. Dou Q, Chen H, Yu LQ, Zhao L, Qin J, Wang DF, Mok VCT, Shi L, Heng PA. Automatic Detection of Cerebral Microbleeds From MR Images via 3D Convolutional Neural Networks. Ieee T Med Imaging 2016;35(5):1182-1195.
3. Liu SF, Utriainen D, Chai C, Chen YS, Wang L, Sethi SK, Xia S, Haacke EM. Cerebral microbleed detection using Susceptibility Weighted Imaging and deep learning. Neuroimage 2019;198:271-282.
4. Chen YC, Villanueva-Meyer JE, Morrison MA, Lupo JM. Toward Automatic Detection of Radiation-Induced Cerebral Microbleeds Using a 3D Deep Residual Network. J Digit Imaging 2019;32(5):766-772.
5. Al-masni MA, Kim WR, Kim EY, Noh Y, Kim DH. Automated detection of cerebral microbleeds in MR images: A two-stage deep learning approach. Neuroimage-Clin 2020;28.
6. Myung MJ, Lee KM, Kim HG, Oh J, Lee JY, Shin I, Kim EJ, San Lee J. Novel Approaches to Detection of Cerebral Microbleeds: Single Deep Learning Model to Achieve a Balanced Performance. J Stroke Cerebrovasc 2021;30(9).
7. Liu SF, Buch S, Chen YS, Choi HS, Dai YM, Habib C, Hub J, Jung JY, Luo Y, Utriainen D, Wang MY, Wu DM, Xia S, Haacke EM. Susceptibility-weighted imaging: current status and future directions. Nmr Biomed 2017;30(4).
8. Tan M, Pang R, Le QV. Efficientdet: Scalable and efficient object detection. 2020. p 10781-10790.
9. Kuijf HJ, de Bresser J, Geerlings MI, Conijn MMA, Viergever MA, Biessels GJ, Vincken KL. Efficient detection of cerebral microbleeds on 7.0 T MR images using the radial symmetry transform. Neuroimage 2012;59(3):2266-2273.
10. Wang SH, Tang CS, Sun JD, Zhang YD. Cerebral Micro-Bleeding Detection Based on Densely Connected Neural Network. Front Neurosci-Switz 2019;13.
11. Hong J, Cheng H, Zhang YD, Liu J. Detecting cerebral microbleeds with transfer learning. Mach Vision Appl 2019;30(7-8):1123-1133.
Figures