0169
BayesTract: Automated machine learning based brain artery segmentation, anatomical prior annotation and feature-extraction in MR Angiography1University of Rochester, Rochester, NY, United States, 2University of Rochester Medical Center, Rochester, NY, United States
Synopsis
Brain arterial-blood-vessels can carry important information regarding cerebrovascular pathogenesis. Due to non-invasiveness, 3D-time-of-flight MR-angiography is widely used to depict arteries in clinical-exams. However, deemed limited for qualitative assessment in clinical setting. Although several post-processing approaches exist such as tool-based-manual segmentation and diameter-marking or deep-learning-based segmentations--these require huge-time and experienced-eyes or large manually-segmented training-sets, making performance variable. Since brain-artery-annotations are anatomically-inspired, anatomical-confidant-points identified with a Bayesian approach can be used to annotate major-brain-arteries, which circumvents the need for large-dataset from learning requirement. The approach will allow clinical evaluations of major-arteries and biomarker identifications for cerebrovascular pathogenesis with limited resource and time.
Introduction
Quantification of cerebral vessels morphological features are important to investigate cerebrovascular pathogenesis1.Due to the non-invasiveness, 3D time-of-flight (TOF) MR angiography (MRA) is widely used technique to depict brain arteries in clinical exams. However, this technique is still limited for qualitative assessment in clinical setting. Although several post-processing approaches exist such as tool-based segmentation2, manual diameter-marking or deep-learning(DL) based segmentations3, 4 , these require huge time and experienced eyes or large manually segmented training-sets, and so performance becomes variable. To reduce the amount of time and resources needed to quantify vessel morphometry thus facilitating implementation in a clinical setting and to identify important biomarkers for cerebrovascular events, we propose a pipeline named “BayesTract”, using unsupervised hidden Markov random field (HMRF)5, 6 for preliminary segmentation and an Anatomy-prior Bayesian scheme for artery identification and morphological feature extraction.Materials & Methods
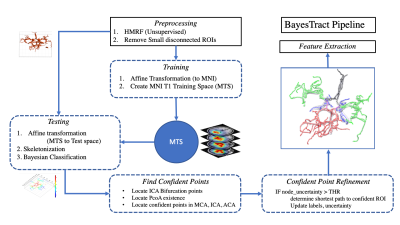
After obtaining informed consent according to the institutional protocol, four healthy control subjects (age 33-46years; 2 for training and 2 for testing) were imaged at a Siemens MAGNETOM Prisma 3T scanner equipped with a 64-channel head coil (receive-only) and body coil transmission, and high-performance gradients (maximum strength 80mT/m) with slew rate of 200mT/m/s. MRI protocols included T1-weighted images using 3D MPRAGE image (TI=962ms, TE/TR=2.34ms/1840ms, 1mm isotropic) for anatomical reference; and TOF MRA images (TE/TR=3.42ms/21ms, in-plane resolution 0.26x0.26 mm2, slice thickness 0.5mm, flip angle=18 degrees).Figure-1 shows the BayesTract pipeline. Briefly, the preprocessing step includes: 1) Extracting the preliminary segmentation map and auto-isolation of relevant largest connected components using HMRF- Expectation Maximization (HMRF-EM)6; 2) Creating MNI-T1 Training Space (MTS) using two manually segmented and labelled dataset verified by an experienced radiologist; 3) FSL MNI T1 0.5 mm atlas is transformed to native space using ANTs, and then create and apply MTS to test subjects. For each test cases, we utilize anatomical confident points from the generated Bayesian uncertainty map with MTS to correctly estimate labels. Final refinement step refines the ROIs where uncertainty is greater than 0.5 and updates the confidence values based on nearest confident points and finally extract features through morphological processing e.g., diameter, tortuosity, vessel length etc. Five types of major arteries considered as classes, are Internal Carotid Artery (ICA), Middle Cerebral Artery (MCA), Posterior Communicating Artery (PComA), Anterior Cerebral Arteries (ACA) and Basilar Artery (BA). Training, testing and uncertainty of labelling with MTS are defined in equations (1-4) as follows
$$ l=(x,y,z)'$$
$$MTS^{l}_{train}=\gamma(\{P^{l}_{ICA},P^{l}_{MCA},P^{l}_{PComA},P^{l}_{ACA},P^{l}_{BA}\})$$
$$MTS^{l}_{test}=max(\sum_{v=l-2}^{l+2}MTS^{v}_{train})$$
$$Uncertainty,u_l=1-(MTS^{l}_{test} \div sum(\sum_{v=l-2}^{l+2}MTS^{v}_{train}))$$
where $$$P^l_X$$$ is Probability of finding $$$X$$$ at location $$$l$$$, $$$X\in \{ICA,MCA,PComA,ACA,BA\}$$$. $$$l$$$ depicts the spatial locations where vessel exists. $$$\gamma$$$ is gamma correction function for the calculated probability maps. Identification of ICA bifurcation points were automated using confident points of MCA, ICA and ACA on both sides which is used in updating label estimation and uncertainty map in final refinement step.
Results & Discussion
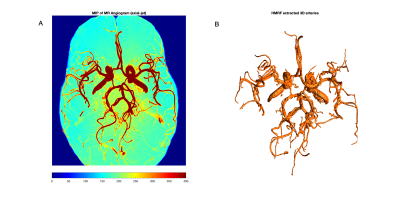
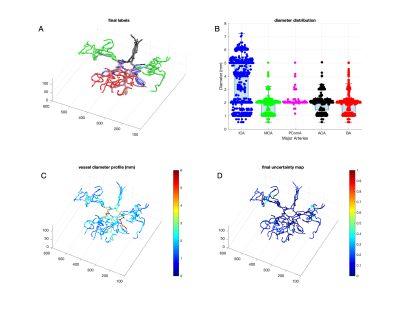
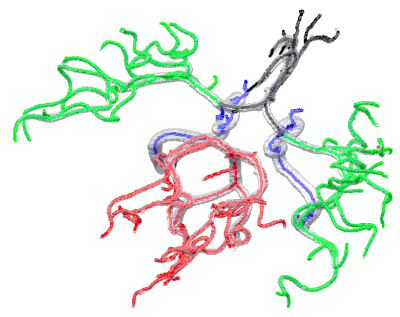
Figure-2 shows an example MRA maximum intensity projection (MIP) image (2A) and corresponding vessel segmentation with HMRF-EM (2B) from a test subject. Note that, HMRF segmentation accuracy is very high (~99%, similar to convolutional DLs5 ) but mainly segments high contrast vessels5. Figure-3 shows initial assessment with for two example artery regions such as (A) Posterior Circulation and (B) Middle Cerebral Artery. Figure-4 shows the final refined (A) labels, (B) diameter distribution, (C) diameter profile and (D) final uncertainty map. Since brain artery annotations are anatomically inspired, this can be utilized as prior by creating on MNI T1 standard space and thereby anatomical points can be identified precisely (uncertainty<0.3) in transformed test space in order to label the major vessels in brain (Figure-4). The preliminary results illustrated with BayesTract looks promising (test accuracy ~99%) and shows practical potential for clinical use. DL approaches need huge computational resources (manually labelled data > 35 subjects, GPU, RAM>20GB)3, 4 which can be a burden for clinical use-cases. Importantly, the accuracy depends on manual train-set labels, leaving room for person-to-person variability. In this proposed approach, anatomical priors ensure accurate classification of vessel using minimum feature space required for the classification, leaving no room for human intervention. We also demonstrate successful labelling of an exceptional case where PComA was absent in the test subject in Figure-5. Further validation and comparison across different datasets/methods are due for this pipeline.Conclusion
The study demonstrates that anatomical confidants can be used to perform automated and accurate identification of major brain arteries without the use of large datasets, making BayesTract useful for clinical evaluations of major arteries and identifying biomarkers for cerebrovascular pathogenesis. The pipeline shows reliable performance in identifying and extracting features from five major arteries (test accuracy ~99%) using only 2 training subjects.Acknowledgements
This work was supported by the National Institutes of Health (Grant Number: R01 AG054328).References
1. Gupta, A., et al., Neuroimaging of cerebrovascular disease in the aging brain. Aging and disease, 2012. 3(5): p. 414.
2. Chen, L., et al., Development of a quantitative intracranial vascular features extraction tool on 3 D MRA using semiautomated open‐curve active contour vessel tracing. Magnetic resonance in medicine, 2018. 79(6): p. 3229-3238.
3. Chen, L., et al. Automated intracranial artery labeling using a graph neural network and hierarchical refinement. in International Conference on Medical Image Computing and Computer-Assisted Intervention. 2020. Springer.
4. Hilbert, A., et al., BRAVE-NET: fully automated arterial brain vessel segmentation in patients with cerebrovascular disease. Frontiers in artificial intelligence, 2020. 3: p. 78.
5. Fan, S., et al., Unsupervised cerebrovascular segmentation of TOF-MRA images based on deep neural network and hidden Markov random field model. Frontiers in neuroinformatics, 2020. 13: p. 77.
6. Wang, Q., HMRF-EM-image: implementation of the hidden markov random field model and its expectation-maximization algorithm. arXiv preprint arXiv:1207.3510, 2012.
Figures