0160
Submillimeter dMRI protocol optimization for accurate in-vivo reconstruction of deep-brain circuitry1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 2Department of Pharmacology and Physiology, University of Rochester School of Medicine, Rochester, NY, United States, 3McLean Hospital, Belmont, MA, United States
Synopsis
The relatively low resolution of conventional in-vivo human dMRI prevents the reconstruction of small axonal bundles (diameter < 3mm). Here we show that diencephalic connections, which are inaccessible at macroscopic resolution (1.5mm), can be reconstructed with unprecedented accuracy at mesoscopic resolution (760μm). We investigate the minimum number of directions needed to achieve this with a state-of-the-art, multi-slab dMRI sequence. We find that a long, multi-session acquisition is necessary. We aim to develop a protocol for manual annotation of these bundles in a small number of high-quality datasets, and thus produce a training set for automated reconstruction in lower-quality dMRI data.
Introduction
Tractography allows white-matter (WM) circuitry to be reconstructed in-vivo. The advances in diffusion MRI (dMRI) acquisition spearheaded by the HCP led to substantial improvements in this reconstruction1,2. However, bundles with diameters in the 1.5-3 mm range cannot be reconstructed, even at the spatial resolution of HCP data (1.5 mm). Several diencephalic connections, that are extremely relevant for psychiatric and motor disorders3, have diameters inferior to 2 mm4. Previous studies have used ex-vivo dMRI to investigate the anatomy of some of these bundles5. However, these studies did not use whole-brain specimens and thus did not reconstruct the entire length of these tracts, and of other neighboring structures. The advent of advanced acquisition and reconstruction strategies6,7,8 has made submillimeter in-vivo MRI feasible. Here we leverage a recently acquired whole-brain in-vivo dMRI dataset acquired at 760 μm9 and sampled at 1260 q-space points across 9 two-hour sessions to investigate the following small diencephalic WM connections: fasciculus retroflexus (FR), stria medullaris (SM), mammillo-thalamic tract (MTh), and mammillo-tegmental tract (MTe). We investigate i) whether sub-millimeter dMRI data can be used to visualize small diencephalic connections of the human brain in-vivo, and ii) the minimum angular resolution (and associated acquisition time) required to obtain accurate reconstructions. We recently showed that automated global probabilistic tractography can be used to transfer anatomical accuracy from higher- to lower-quality dMRI data10,11. We envision using the optimized protocol presented here to acquire submillimeter dMRI data in a small set of training subjects, and use them to inform reconstruction of the same tracts from lower-quality dMRI scansMethods
Data: Data were acquired on the MGH-USC 3T Connectom Scanner using an SNR-efficient simultaneous multi-slab imaging technique (gSlider-SMS)7,8 across 9 two-hour sessions (max gradient amplitude: 180 mT/m; slew rate: 125 T/m/s, gSlider factor: 5, MB factor: 2, R: 3, TR/TE: 3500/75 ms, matrix: 290x288, PE: AP). 2808 dMRI volumes were acquired (144 b=0 s/mm2, 420 b=1000 s/mm2, 840 b=2500 s/mm2, along with their paired reversed PE volumes). Processing: We used pre-processed, publicly available data12,13. We investigated the effect of angular resolution on tractography, by generating 4 uniformly sampled subsets of the 1,262AP+1,262PA directions: i) 630AP+630PA, ii) 420AP+420PA, iii) 630AP, iv) 420AP. For each dataset, we fit fODFs using MSMT-CSD14 in MRtrix315. We performed probabilistic tractography with whole-brain dynamic seeding to obtain 20M streamlines. Dissections: Tracts were annotated manually by author CM in TrackVis using the full dataset. Tracts were defined as follows16,17. FR: coursing from the interpeduncular nucleus to the habenula, medial to the red nucleus. SM: coursing from the septal area to the habenula, superior to the thalamic dorsomedial nucleus. MTh: coursing from the mammillary bodies to the anterior nucleus of the thalamus. MTe: coursing from the mammillary bodies to the midbrain. For each subsampled dataset, tracts were extracted using the same ROIs. Accuracy was quantified by the Dice coefficient (DC) between tracts obtained from the subsampled and full datasets.Results
Fig.1 shows the importance of submillimeter spatial resolution for the reconstruction of small diencephalic connections. At 1.5x1.5x1.5 mm, bigger bundles (i.e., fornix) can be reconstructed correctly but the SM and MTe are only partially reconstructed, while the FR and MTh are impossible to reconstruct (Fig.1.). At 0.76x0.76x0.76 mm, all bundles can be delineated with high anatomical accuracy, as evidenced by the high topographical agreement with the Duvernoy atlas (Fig.2). Fig.3,4 show the effect of reducing the angular resolution. With 630AP+630PA directions, we retain reconstruction accuracy for most tracts (DC > 60%). With 420AP+420PA directions, reconstruction accuracy is diminished. Further reduction in the number of directions (630AP, 420AP) leads to a significant reduction in both volume and accuracy of the reconstructed tracts (Fig.3,4). As angular resolution decreases, fODFs become noisier and orientations less coherent (Fig.5). This has a greater effect on smaller bundles that are characterized by lower signal-to-noise ratio or substantial partial voluming with gray matter, like the SM (Fig.5).Discussion and Conclusions
Our results show that submillimeter dMRI can be used to obtain highly accurate reconstructions of small diencephalic WM connections in-vivo. Anatomical accuracy is preserved when using half of the gradient directions contained in the original protocol, reducing the acquisition time from ~15 to ~7.5 hr. Such a protocol is still impractical for large-scale studies. In recent work, we showed that global probabilistic tractography with anatomical priors can be trained on high-quality dMRI data and used to perform accurate, automated reconstruction in lower-quality data1,2. We envision using the submillimeter dMRI protocol presented here to perform manual annotation of these WM tracts in a small set of training subjects. These data can then be used either to inform or to validate tractography at lower, more practical spatial resolutions10. Building an atlas of deep-brain pathways at submillimeter resolution would also be valuable for the clinical interventions that target these pathways, including deep brain stimulation18 and MRI-guided focused ultrasound19.Acknowledgements
This work was supported by the National Institute for Biomedical Imaging and Bioengineering [R01-EB021265, U01-EB026996] and the National Institute for Mental Health [U01-MH108168, R56-MH121426]. It was carried out at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41-EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. The work used data acquired with support from the NIH Blueprint for Neuroscience Research [U01-MH093765; part of the multi-institutional Human Connectome Project, T90DA022759/R90DA023427] and relied on instrumentation supported by the NIH Shared Instrumentation Grant Program [S10RR023401, S10RR019307, S10RR023043].References
1.Maffei C., Sarubbo S., Jovicich J. Diffusion-based tractography atlas of the human acoustic radiation. Sci. Rep. 2019;9.
2. Mcnab J.A., Edlow B.L., Witzel T., Huang S.Y., Bhat H., Heberlein K., Feiweier T., Liu K., Keil B., Cohen-Adad J., Tisdall M.D., Folkerth R.D., Kinney H.C., Wald L.L.. The Human Connectome Project and beyond: Initial applications of 300 mT/m gradients. Neuroimage. 2013;80:234-245.
3.Fakhoury M. The habenula in psychiatric disorders: More than three decades of translational investigation. Neurosci Biobehav Rev. 2017;83:721-735.
4.Gallay M.N., Jeanmonod D., Liu J., Morel A.Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery. Brain structure & function. 2018; 212(6):443–463.
5. Oishi K, Mori S, Troncoso JC, Lenz FA. Mapping tracts in the human subthalamic area by 11.7T ex vivo diffusion tensor imaging. Brain Struct Funct. 2020;225(4):1293-1312.
6. Keil, B. et al. A 64‐channel 3T array coil for accelerated brain MRI. Magn. Reson. Med. 2013; 70, 248–258.
7.Setsompop, K. et al. High‐resolution in vivo diffusion imaging of the human brain with generalized slice dithered enhanced resolution: Simultaneous multislice (gSlider-SMS). Magn. Reson. Med. 2018; 79, 141–151.
8.Wang, F. et al. Motion‐robust sub‐millimeter isotropic diffusion imaging through motion corrected generalized slice dithered enhanced resolution (MC-gSlider) acquisition. Magn. Reson. Med. 2018; 80, 1891–1906.
9.Wang F., Dong Z., Tian Q. et al. In vivo human whole-brain Connectom diffusion MRI dataset at 760 µm isotropic resolution. Sci Data. 2021; 8, 122.
10. C. Maffei, C. Lee, M. Planich, M. Ramprasad, N. Ravi, D. Trainor, Z. Urban, M. Kim, R.J. Jones, A. Henin, S.G. Hofmann, D.A. Pizzagalli, R.P. Auerbach, J.D.E. Gabrieli, S. Whitfield-Gabrieli, D.N. Greve, S.N. Haber, A. Yendiki. Using diffusion MRI data acquired with ultra-high gradients to improve tractography in routine-quality data. bioRxiv 2021.06.28.450265
11.Yendiki A., Panneck P., Srinivasan P., Stevens A., Zöllei L., Augustinack J., Wang R., et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Frontiers in Neuroinformatics. 2011; 5: 23.
12.Wang, F. et al. Data from: In vivo human whole-brain Connectom diffusion MRI dataset at 760 μm isotropic resolution (PART I). Dryad https://doi.org/10.5061/dryad.nzs7h44q2 (2021).
13. Wang, F. et al. Data from: In vivo human whole-brain Connectom diffusion MRI dataset at 760 μm isotropic resolution (PART II). Dryad https://doi.org/10.5061/dryad.rjdfn2z8g (2021).
14.Jeurissen B., Tournier J. D., Dhollander T., Connelly A., Sijbers, J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage. 2014; 103, 411-426.
15.Tournier J. D., Smith R., Raffelt D., Tabbara R., Dhollander T., Pietsch, M., [...], Connelly A. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage. 2019; 202, 116137.
16.Duvernoy's Atlas of the Human Brain Stem and Cerebellum. AJNR Am J Neuroradiol. 2009;30(5):e75.
17.Roman E., Weininger J., Lim B., et al. Untangling the dorsal diencephalic conduction system: a review of structure and function of the stria medullaris, habenula and fasciculus retroflexus. Brain Struct Funct. 2020;225(5):1437-1458.
18. Li N., Baldermann J.C., Kibleur A. et al. A unified connectomic target for deep brain stimulation in obsessive-compulsive disorder. Nat Commun. 2020; 11, 3364 ().
19. Tian Q., Wintermark M., Jeffrey E. W., et al. Diffusion MRI tractography for improved transcranial MRI-guided focused ultrasound thalamotomy targeting for essential tremor. Neuroimage Clin. 2018;19:572-580.
Figures
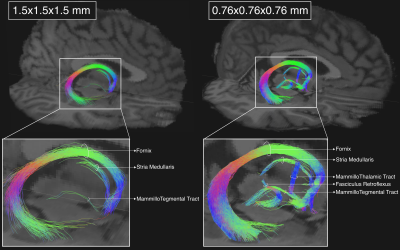
Fig 1. Effect of spatial resolution. Tractography reconstructions of the right-hemisphere diencephalic white matter connections for one of the MGH-USC HCP subjects (MGH_1006) acquired at 1.5x1.5x1.5 mm spatial resolution (left), and for the submillimeter data acquired at 0.76x0.76x0.76 mm (right). The mammillo-thalamic tract and the fasciculus retroflexus are only visible at 0.76x0.76x0.76 mm.
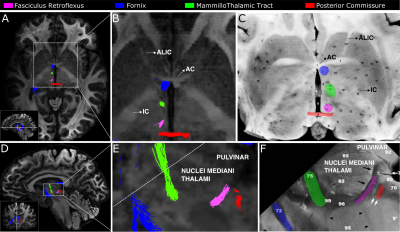
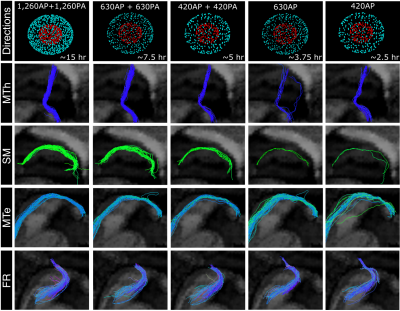
Fig 3. Effect of angular resolution. Tractography of the right mammilllo-thalamic tract (MTh), mammillo-tegmental tract (MTe), stria medullaris (SM), and fasciculus retroflexus (FR) from the full dMRI dataset and each of the sub-sampled datasets. Number of gradient directions and approximate time required for their acquisition are also shown (top row).
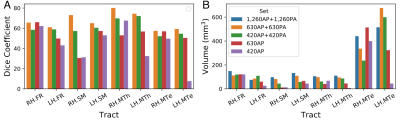
Fig 4. Quantitative evaluation. A) Spatial overlap, measured by the Dice coefficient, between tracts reconstructed using the full dataset and those reconstructed using each one of the sub-sampled datasets. B) Volume of tracts reconstructed from the full dataset and each of the sub-sampled datasets.
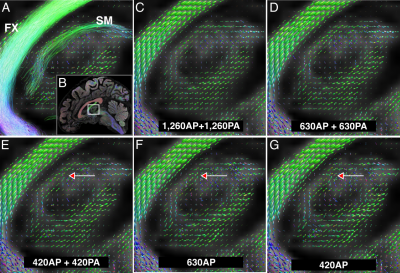
Fig 5. Comparison of fiber orientation distribution functions (fODFs). A) Sagittal view showing a cross-section of the right stria medullaris (SM) and fornix (FX), along with the reconstructed fODFs for that slice. The location of the magnification box is shown in B. C-G) Reconstructed fODFs for the full dataset (C) and each of the sub-sampled datasets (D-G). Arrows point to SM fODFs getting noisier as the number of gradient directions decreases.