0156
Ultra-high-resolution multi-parametric imaging on rats using MULTIPLEX at 9.4T1UIH America, Inc., Houston, TX, United States, 2Wuhan United Imaging Life Science Instrument Co., Ltd., Wuhan, China
Synopsis
In this work, we report the initial experience of a state-of-the-art single-scan, high-resolution multi-parametric method, i.e. MULTIPLEX, on a 9.4T animal system. Ultra-high-resolution multi-parametric images with 0.12mm isotropic voxels were achieved on ex-vivo rat brain with atlas level anatomical details, while high resolution (0.1x0.1x0.4mm3) results with sufficient SNR were achieved with in-vivo rat brain.
Introduction
It is challenging to perform single-scan multi-parametric imaging at ultra-high fields (e.g. ≥7T) due to factors such as elevated specific-absorption-rate and peripheral nerve stimulation, longer T1 and shorter T2/T2* relaxometries, increased B1+ inhomogeneity and chemical shifts, to name a few. As the result, reports on multi-parametric small animal imaging at ultra-high-fields are rather limited, and all with very limited imaging resolution (e.g. 2D imaging with 0.23mm pixels and 1.5mm slices)1,2.Recently, a 3D high-resolution single-scan multi-parametric imaging method, namely MULTIPLEX3, was proposed and demonstrated on 3T. The MULTIPLEX method features in dual-TR, dual-FA and multi-echo GRE acquisition. In principle, GRE-based methods perform better at higher fields in terms of T1 , PD, susceptibility, SNR and scan times, yet without limitations on resolution and SAR. Therefore, this work aims to perform the initial evaluation of the MULTIPLEX method for ultra-high-resolution imaging on a 9.4T animal system.
Methods
MR Instruments: All MR experiments were performed in a horizontal 30cm-bore 9.4T system (uMR 9.4T, United Imaging Healthcare, Wuhan, China) with gradient performance of 1000mT/m and 10000T/m/s. For ex-vivo experiments, a quadrature volume transceiver coil (24mm inner-diameter) was used. For in-vivo experiments, a quadrature volume transmitter coil (86mm inner-diameter) and a 4-channel rat head surface receiver coil were used.Ex-vivo rat brain preparations: The ex-vivo rat brain preparation was approved by local IRB, and detailed as previously described4. Before all MR scans, ex-vivo brains were placed in Dulbecco's phosphate-buffered saline for at least two days before secured with Fomblin oil.
Animal preparation: With local IRB approval, one healthy adult rat was induced anesthesia using 5% isoflurane and maintained under 1.5% for MRI scan thereafter. Throughout the entire procedure, the rat was monitored (SA Instruments) and well-maintained for rectal temperature at 37°C and respiration rate at ~60bpm.
MR Imaging Parameters: The ex-vivo MULTIPLEX parameters were: coronal FOV=24x16mm2, matrix size=208x139x96, voxel size=0.115x0.115x0.120mm3, TR1/TR2=7.6/38.4ms, FA1/FA2=4°/24°, 5 echoes with TE=2.79~26.99ms, bandwidth=150Hz/px, and TA=10.9hr with NEX=32 and full k-space sampling.
The in-vivo MULTIPLEX parameters were: axial plane with FOV=20x20mm2, matrix size=208x208x72, voxel size=0.096x0.096x0.400mm, TR1/TR2 = 3/15ms, FA1/FA2=4°/16°, 5 echoes with TE=1.10~9.90ms, bandwidth = 450Hz/px, and TA=54min with NEX = 8 and full k-space sampling.
Data Reconstruction: Dicom images of the MULTIPLEX scans were directly reconstructed by both scanners' inline reconstruction pipeline.
Results
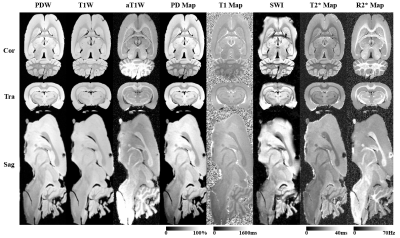
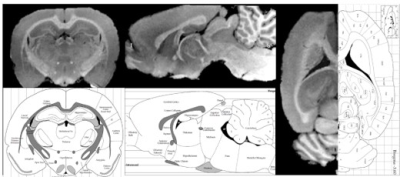
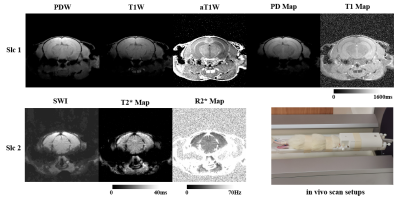
Transectional ex-vivo rat brain images are shown in Figure 1, showing typical MULTIPLEX images of PDW, T1W, aT1W, SWI and PD/T1/T2*/R2* maps. Figure 2 compares the ex-vivo aT1W images to a rat brain atlas5 to illustrate the fine anatomical details. Figure 3 shows the in-vivo images as well as the scan setups.Discussion
In this work, we have presented the very first MULTIPLEX results at a 9.4T animal system, showing several interesting findings.Firstly, both the ex- and in-vivo results served as direct proofs of the MULTIPLEX method on achieving ultra-high-resolution multi-parametric imaging at 9.4T. At such a high field strength, the fast decaying FID signals will limit the SPIRAL and EPI resolution, while the longer tissue T1 and higher SAR level will respectively increase the scan time of IR- and SE-based method. However, these factors actually worked to the benefit of the GRE-based MULTIPLEX method, where lower FA can be used to achieve proper T1 weightings and SNR, thus further lowering the already low SAR level and ameliorating B1 inhomogeneity. Also, faster scans can be achieved by using shorter echo spacing (i.e. higher readout bandwidth) to accommodate the faster T2* decay. Furthermore, GRE readouts support 3D acquisition for real high resolution imaging, a feature currently not possible on certain multi-parametric methods6,7.
As illustrated in Figures 1&2, with the 0.12mm isotropic ultra-high-resolution, the ex-vivo images can be viewed from arbitrary cross-sectional planes for direct anatomy delineation with atlas level definition (Figure 2), such as cerebral cortex, corpus callosum, amygdala, hippocampus, olfactory bulb, thalamus, hypothalamus, midbrain, pons, medulla oblongata, spinal cord, cerebellum, caudate putamen, with extension to ventricles and even some other small structures like fornix, optic tract and anterior/posterior commissures.
Secondly, T1W contrasts of both ex- and in-vivo scans were quite weak (Fig.2) or even reversed (Fig.1), although sufficiently high FA (i.e. 24° and 16°) were already used. This is likely the results of non-T1 effects like proton density and T2* relaxation. However, the MULTIPLEX aT1W and T1 maps reliably enhanced and quantified the T1 effects, showing excellent anatomical details3 with expected contrasts.
Thirdly, with the multi-dimensional integration (MDI) method8, the resultant aT1W, T1 maps and T2*/R2* maps from MULTIPLEX are generally spatially uniform, as clearly shown in the in-vivo images . This was the result of removal of coil sensitivity, which was strongest at the top of the head, during MDI calculation8.
On the other hand, the residual inhomogeneity in the in-vivo T1 map suggested the B1+ map9 requires further tailoring to accommodate the ultra-high-field small FOV scenario. Also the MULTIPLEX protocol should be further optimized for better T1 contrasts and SNR for in-vivo small animal imaging. Lastly, better receiver coils with round-the-head design can further support acquisition acceleration techniques for practical live animal studies.
Conclusion
In summary, we have demonstrated the ultra-high-resolution imaging capacity of the MULTIPLEX method at a 9.4T animal system, suggesting its potential for single-scan multi-parametric preclinical studies.Acknowledgements
No acknowledgement found.References
1. Anderson CE, Johansen M, Erokwu BO, et al. Dynamic, Simultaneous Concentration Mapping of Multiple MRI Contrast Agents with Dual Contrast - Magnetic Resonance Fingerprinting. Sci Rep 2019;9(1):19888.
2. Gu Y, Wang CY, Anderson CE, et al. Fast magnetic resonance fingerprinting for dynamic contrast-enhanced studies in mice. Magn Reson Med 2018;80(6):2681-2690.
3. Ye Y, Lyu J, Hu Y, Zhang Z, Xu J, Zhang W. MULTI-parametric MR imaging with fLEXible design (MULTIPLEX). Magn Reson Med 2021.
4. Gage GJ, Kipke DR, Shain W. Whole animal perfusion fixation for rodents. J Vis Exp 2012(65).
5. Paxinos G, Watson C. Paxino's and Watson's The rat brain in stereotaxic coordinates. Amsterdam ; Boston: Elsevier/AP, Academic Press is an imprint of Elsevier: 2014. 1 volume (unpaged) p.
6. Gao Y, Chen Y, Ma D, et al. Preclinical MR fingerprinting (MRF) at 7 T: effective quantitative imaging for rodent disease models. NMR Biomed 2015;28(3):384-394.
7. Warntjes JB, Leinhard OD, West J, Lundberg P. Rapid magnetic resonance quantification on the brain: Optimization for clinical usage. Magn Reson Med 2008;60(2):320-329.
8. Ye Y, Lyu J, Sun W, et al. A multi-dimensional integration (MDI) strategy for MR T2 * mapping. NMR Biomed 2021;34(7):e4529.
9. Yarnykh VL. Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med 2007;57(1):192-200.
Figures