0154
Probing Mitochondrial Function in Intact Fetal Brains with Ungated 4D Oxy-wavelet MRI in an Irradiation Injury Mouse Model1Cedars Sinai Medical Center, Los Angeles, CA, United States, 2Developmental Biology, University of Pittsburgh, Pittsburgh, PA, United States, 3Biomedical Engineering, University of Pittsburgh, Pittsburgh, PA, United States, 4Pediatrics, University of Pittsburgh, Pittsburgh, PA, United States, 5Radiation Oncology, University of Pittsburgh, Pittsburgh, PA, United States
Synopsis
A novel motion-and-time resolved 4D oxy-wavelet MRI (4D-fMRI acquired with oscillating hypoxia challenges, analyzed by a continuous wavelet transform mimicking experimental oscillations) can acquire fetal MRI with high spatiotemporal resolution and can probe mitochondrial functions in live fetal brains. 4D oxy-wavelet MRI outcomes were validated with Oroboros mitochondrial function assays and correlated with mitochondrial targeting drug JP4-039 in a fetal irradiation injury mouse model. The mouse fetuses showed poor 4D oxy-wavelet outcomes had poor mitochondrial functions and vice versa. Furthermore, an automated time-frequency analysis scheme can correctly differentiate normal vs irradiated fetuses, paving the way for future AI-based automatic diagnosis.
INTRODUCTION
Mitochondrial dysfunction is a critical element for wide ranges of brain pathological conditions, such as traumatic brain injury and neurodegenerative diseases; thus, mitochondria are emerging as promising therapeutic targets for brain diseases. However, a non-invasive means to probe mitochondrial functions in intact brains, especially in fetal brains, is lacking. Fetal brain MRI is challenging due to non-predictable fetal movement and small anatomical structures, and more importantly, greatly limited by resolution-mismatch of anatomical and functional scans. Co-registration of low-resolution dynamic blood-oxygenation dependent (BOLD) functional MRI signals onto high-resolution anatomical MRI can result in incorrect mapping of functional activities, exacerbated by fetal motions.We have established a motion-and-time resolved 4D functional MRI (4D-fMRI)[1, 2] capable of 3D isotropic MRI time-series to simultaneously capture fast dynamic BOLD signals in the same anatomical scan with both high-spatial and high-temporal resolution (voxel size: 0.00047 mm3, frame rate: 14ms) using sub-Nyquist sparse sampling. We leverage the fact that the acute adaptation to hypoxia, the fetal “brain sparing” capability[3-5], requires mitochondria to probe mitochondrial functions in fetal brains, called 4D oxy-wavelet MRI (4D-fMRI in conjunction with oscillating hypoxia challenges, analyzed by a continuous wavelet transform mimicking experimental oscillations). We tested its capability in a fetal mouse irradiation injury model.
METHODS
4D Fetal Oxy-wavelet MRI: Our method uses a hybrid low-rank [6] and sparse [7] model to measure a dynamic BOLD image $$$\rho(\mathbf{r},t)$$$ (for spatial position $$$\mathbf{r}$$$ and time $$$t$$$) from undersampled $$$(\mathbf{k},t)$$$ -space data. The low-rank model expresses the image as the outer product of a set of $$$L$$$ basis images $$$\{\psi_\ell(\mathbf{r})\}_{\ell=1}^L$$$ and $$$L$$$ temporal functions $$$\{\varphi_\ell(t)\}_{\ell=1}^L$$$:$$\rho(\mathbf{r},t)=\sum_{\ell=1}^L\psi_\ell(\mathbf{r})\varphi_\ell(t)$$ When enforced during image reconstruction, this model exploits correlation of images over time [8]; transform sparsity [9] of $$$\{\psi_\ell(\mathbf{r})\}_{\ell=1}^L$$$ can additionally be enforced for even higher acceleration. This allows for fMRI with high spatiotemporal resolution; furthermore, it can assess oxygen attenuation during the same single scan. 4D-fMRI was acquired with a 7-Tesla preclinical scanner (Bruker Biospec USR 70/30) with a 35-mm quadrature volume coil, FOV=4.5cm×3cm×2cm, isotropic voxel size 120μm×120μm×120μm, FA=10°, TR/TE=8.3ms/4.5ms, scan time=40min. During acquisition, short bursts of 3-min hypoxia (10% O2) interleaved with 3-min hyperoxia (100% O2) were supplied via a nose cone to pregnant females.We also developed an automated time-frequency analysis scheme for 4D oxy-wavelet MRI. Our BOLD signal is event-driven, following the oscillations of the hypoxia challenge; the event waveform resembles a square wave (Fig.3). Convolving the BOLD signal by template event waveforms at several oscillation frequencies will produce a wavelet-like feature space revealing various details.
Animal Model: Pregnant female C57BL6/J mice (n=10) were subjected to 3Gy full-body irradiation on embryonic day E13.5. One day later on E14.5, half received mitochondrial targeting mitochondrial targeted GS-nitroxide radiation mitigator, JP4-039[10-12], whereas the other half received saline as controls. The pregnant females were imaged on E16.5 with the 4D oxy-wavelet MRI.
Ex vivo mitochondrial functional assay: After MRI, freshly harvested fetal brain homogenates were subjected to the Oroboros respirometry for mitochondrial oxygenation consumption. The tricarboxylic acid (TCA) cycle substrates and inhibitors for Complex I and II were added in a stepwise fashion to evaluate relative contributions of each complex.
RESULTS
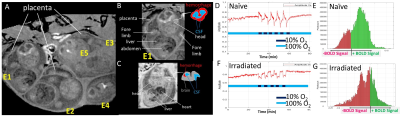
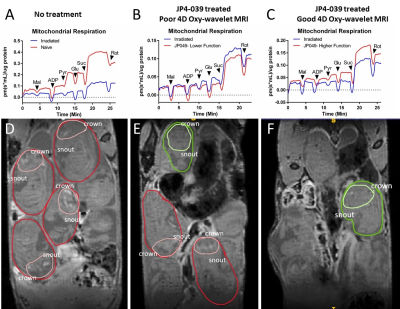
After 3Gy irradiation on embryonic day E13.5, the pregnant female mice (~8 fetuses per pregnancy) were randomized to receive mitochondria-targeted drug JP4-039 on E14.5. 4D-fMRI on E16.5 showed that the irradiated embryos exhibited ventriculomegaly with excessive cerebrospinal fluid (CSF), (Fig.1A-C, grey), thinning of the cortex, and cerebral hemorrhage (Fig.1A-C hyperintensity and drawing red). Furthermore, we have successfully probed fetal brain mitochondrial function in vivo by transient short bursts (3 minutes) of hypoxia (10% O2) via mother’s inhalation in (Fig.1D,F) and validated with Oroboros respirometry (Fig.2A-C). Naïve fetal brains (Fig.1D,E) with intact mitochondrial functions can overcome the bursts of hypoxia (Fig.1D dark blue periods) to quickly adjust to an even higher oxygenation state after initial transient BOLD signal drop, indicating intact “brain sparing” capability. Most of the voxels showed positive BOLD signals (Fig.1E,green) during hypoxia. On the other hand, irradiated fetal brains (Fig.1FG) with mitochondrial dysfunctions showed decreased BOLD signal (Fig.1F) throughout hypoxia, indicating an inability to respond to a hypoxia challenge (Fig.1G,red).The irradiated fetal brains showed compromised mitochondrial respiration measured by Oroboros respirometry (Fig.2A-C). Mitochondrial targeting drug JP4-039[10-12] could partially rescue some of the fetal brains. The ones showed improved function with 4D oxy-wavelet MRI (Fig.2D-F, green outlines) showed improved mitochondrial respiration (Fig.2C); the ones did not show improvement with 4D oxy-wavelet MRI (Fig.2D-F, red outlines) showed little improvement with Oroboros (Fig.2B).
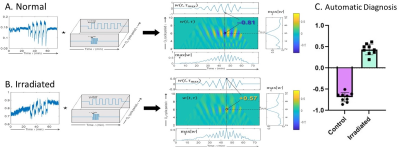
Fig.3 shows that for automated oxy-wavelet analysis, the sign of the peak value reveals the direction of oxygen response: a positive peak indicates a positive correlation with the oxygen cycling (tissue hypoxia when external oxygen is decreased), whereas a negative peak indicates opposite behavior (brain sparing wherein tissue over-corrects for external oxygen decreases by adjusting to an even higher oxygenation). This allows automatic computerized detection of passive brain BOLD responses in the injured brains (positive correlation) vs. active compensation in the normal brains (negative correlation), paving the way for future artificial intelligence (AI)-based diagnosis strategies.
CONCLUSION
The single 4D oxy-wavelet MRI scan can probe both structure and mitochondrial functions simultaneously in fetal mouse brains.Acknowledgements
MSC and YLW are supported by funding from NIH-R21-EB023507, AHA-18CDA34140024, and DoD-W81XWH1810070.References
1. Christodoulou, A.G., et al. Fetal Brain-Heart-Placental Interactions with Acute Hypoxia Challenge in Genetic Mouse Models of Hypoplastic Left Heart Syndrome with in utero 4D Dynamic MRI. in ISMRM International Society of Magnetic Resonance in Medicine. 2019. Montreal, QC, Canada.
2. Christodoulou, A.G., et al., 4D Real-time BOLD MRI in Genetically Engineered Mouse Brains with Acute Hypoxia Challenge, in International Soceity of Magnetic Resonance in Medicine (ISMRM). 2019: Montreal, QC, Canada.
3. Lee, P., N.S. Chandel, and M.C. Simon, Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol, 2020. 21(5): p. 268-283.
4. Shapiro, J., et al., BOLD-MRI demonstrates acute placental and fetal organ hypoperfusion with fetal brain sparing in response to phenylephrine but not ephedrine. Placenta, 2020. 90: p. 52-57.
5. Cahill, L.S., et al., Fetal brain sparing in a mouse model of chronic maternal hypoxia. J Cereb Blood Flow Metab, 2019. 39(6): p. 1172-1184.
6. Christodoulou, A.G., et al., High-resolution cardiovascular MRI by integrating parallel imaging with low-rank and sparse modeling. IEEE Trans Biomed Eng, 2013. 60(11): p. 3083-92.
7. Zhao, B., et al., Image reconstruction from highly undersampled (k, t)-space data with joint partial separability and sparsity constraints. IEEE Trans Med Imaging, 2012. 31(9): p. 1809-20.
8. Liang, Z.-P., Spatiotemporal imaging with partially separable functions. Proc IEEE Int Symp Biomed Imaging, 2007: p. 988-991.
9. Lustig, M., D. Donoho, and J.M. Pauly, Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med, 2007. 58(6): p. 1182-95.
10. Rajagopalan, M.S., et al., The mitochondria-targeted nitroxide JP4-039 augments potentially lethal irradiation damage repair. In Vivo, 2009. 23(5): p. 717-26.
11. Frantz, M.C. and P. Wipf, Mitochondria as a target in treatment. Environ Mol Mutagen, 2010. 51(5): p. 462-75.
12. Epperly, M.W., et al., Effectiveness of Analogs of the GS-Nitroxide, JP4-039, as Total Body Irradiation Mitigators. In Vivo, 2017. 31(1): p. 39-43.
Figures