0147
Clinical validation of ADC thresholds established by the ECOG-ACRIN A6702 trial to reduce unnecessary biopsies in breast MR screening exams1Radiology, University of Washington, Seattle, WA, United States, 2Radiology, Kangbuk Samsung Hospital, Seoul, Korea, Republic of
Synopsis
Numerous single-site studies have established that diffusion-weighted MRI (DWI) can improve breast MRI performance, but clinical implementation has been limited by lack of standardization. The ECOG-ACRIN multicenter A6702 trial determined two pre-specified ADC thresholds from standardized acquisition that could reduce unnecessary biopsies by up to 36%. Since lesion ADCs were computed using centralized offline analysis, whether similar performance could be achieved in clinical settings is not yet established. We demonstrate that implementing the ADC threshold specified by the ECOG-ACRIN trial in a clinical breast imaging setting could provide high sensitivity and potentially reduce unnecessary biopsies for screening breast MRI.
Introduction
Dynamic Contrast Enhanced (DCE) MRI is a highly sensitive tool for breast cancer detection but has moderate specificity [1]. Using centralized measurements and image quality/lesion evaluability criteria, the ECOG-ACRIN A6702 multicenter trial demonstrated diffusion weighted imaging (DWI) can reduce the biopsy rate of DCE MRI while maintaining sensitivity, and identified an optimal apparent diffusion coefficient (ADC) threshold to distinguish benign from malignant lesions (1.53x10-3 mm2/s) with potential reduction of 36% unnecessary (benign) biopsies and 20.9% of biopsies overall [2]. An important next step in translating clinical trial results into routine practice settings includes validation of DWI measurements in a real-world clinic environment with limited software tools for lesion quantitation available to radiologists. The purpose of this study was to validate the ADC thresholds established by the ECOG-ACRIN A6702 trial in a clinical environment and to compare the diagnostic performance obtained using commercially-available clinical versus research software tools to measure lesion ADCs.Materials and Methods
Subjects: In this IRB approved study, screening breast MRI examinations (January 2016-January 2019) with a lesion recommended for biopsy (BI-RADS 4 or 5 assessments) were evaluated. Over the study timeframe, interpreting radiologists prospectively recorded lesion ADC measures using the clinical PACS workstation (Centricity RA1000, GE Healthcare). During the study period, ADC was not used to determine BI-RADS. MRI Acquisition: Imaging was performed on a 3T clinical scanner (Achieva, Philips Healthcare, Best, Netherlands) using a 16-channel breast coil. The breast MRI protocol included: T2-weighted, DWI, and DCE-MRI sequences. Using the A6702 trial protocol, DWI was acquired with TR/TE=5043/60 ms, FOV=360x360 mm2, voxel size=1.8×1.8×4 mm3, NSA=2, SPAIR with gradient reversal fat suppression, b=0/100/600/800/1000s/mm2, 30 slices, and 4:33 min scan time.Image Analysis: Lesion ADC values were collected 3 ways: 1) prospectively at the time of initial interpretation (‘ClinicalProspective’), 2) retrospectively by a single trained radiologist using a newer clinical workstation (Centricity Universal Viewer, GE Healthcare) (‘ClinicalRetrospective’) and 3) by a researcher using dedicated software utilized in the ECOG-ACRIN A6702 trial (‘Research’). Scanner-generated ADC maps with b=0/100/600/800 s/mm2 were used for ClinicalProspective and ClinicalRetrospective lesion ADC measurements. The interpreting radiologist defined regions-of-interest (ROIs) on these ADC maps and specified the lesion type as part of routine BI-RADS clinical reporting. For Research measures, custom software developed in MATLAB (MathWorks, Natick, MA) was used to generate ADC maps using b=0/800 s/mm2 images and monoexponential decay model (following EUSOBI standardization guidelines [3]) and lesion ROIs were drawn on b=800 s/mm2 images using a semi-automated segmentation tool and propagated to calculated ADC maps as in the A6702 trial [2]. Prospective measurements were performed prior to biopsy at the time of clinical MRI interpretation, and retrospective measurements were performed blinded to biopsy outcomes.
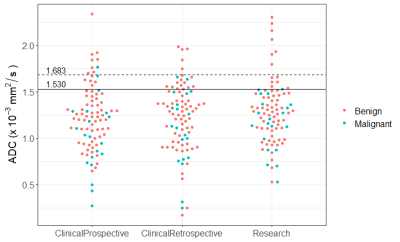
Statistical Analysis: ADC values of malignant and benign lesions were compared by unpaired t-test. Diagnostic performance for differentiating benign and malignant lesions was assessed by receiver operating characteristics (ROC) curve analysis, and effect on biopsy rate and sensitivity was evaluated for the two prespecified A6702-identified thresholds (1.53x10-3 mm2/s and a more conservative 10%-inflated threshold 1.683x10-3 mm2/s [2]).
Results
A total of 119 BI-RADS 4 or 5 lesions on screening MRIs in 104 women were identified over the study timeframe; 89 (69 benign, 20 malignant) lesions in 77 women had a prospectively recorded ADC at the time of clinical interpretation, which constituted the study cohort (Table 1).Diagnostic performance for distinguishing benign and malignant lesions:
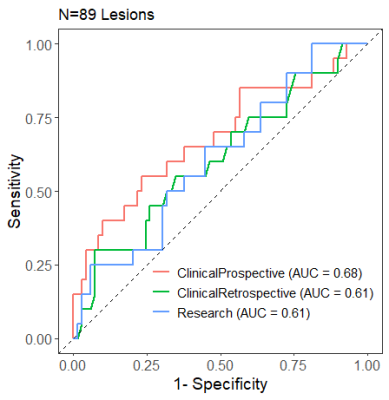
Mean ADC values of benign lesions were generally higher than malignancies, but significantly different only for ClinicalProspective measures (Table 2). Area under the curve (AUC) for predicting malignancy was 0.68 (95% CI 0.60-0.76), 0.61 (95% CI 0.51-0.71), and 0.61 (95% CI 0.51-0.71), respectively for ClinicalProspective, ClinicalRetrospective and Research evaluations (Figure 1). Application of the lower ADC threshold, where lesions with ADC >1.53 x10-3 mm2/s would not undergo biopsy, resulted in 90%-100% sensitivity (2 DCIS lesions were missed in ClinicalProspective and ClinicalRetrospective evaluations but identified by Research evaluation) and 19%-25% benign biopsy reduction (Table 3). Stratifying results by lesion type demonstrated 100% sensitivity for masses and 78%-100% sensitivity for non-masses. The biopsy reduction rate was generally greater for non-masses (range:18-36%) than masses (range:15-20%). Application of the higher ADC cutoff of 1.683 x10-3 mm2/s resulted in similar sensitivity but less reduction in biopsies (Table 3), as shown in Figure 2.
Discussion and Conclusion
Our findings demonstrate that appropriate prospective application of the ADC thresholds identified in the prior ECOG-ACRIN A6702 multicenter trial at our institution would prevent up to 25% of benign biopsies and maintain high sensitivity (≥90%) for breast cancer detection. Performance varied by lesion type, with higher sensitivity in masses and greater biopsy reduction in non-masses. Despite the unavailability of tailored software tools for measuring lesion ADC values in the clinical setting, the potential reduction in biopsy rate was comparable to that achieved in the research environment. Of note, implementing lesion evaluability/image quality criteria into clinical ADC assessments was not performed in this study but would likely improve performance further, as demonstrated in the A6702 trial [4]. Overall, this validation study provides further evidence that breast DWI may be successfully integrated into clinical breast MRI interpretations.Acknowledgements
Supported by NIH/NCI research grant R01CA207290 and the Safeway Foundation.References
1. Peters NH, Borel Rinkes IH, Zuithoff NP, et al. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008 Jan;246(1):116-24.
2. Rahbar H, Zhang Z, Chenevert TL, et al. Utility of Diffusion-weighted Imaging to Decrease Unnecessary Biopsies Prompted by Breast MRI: A Trial of the ECOG-ACRIN Cancer Research Group (A6702). Clin Cancer Res. 2019 Mar 15;25(6):1756-1765.
3. Baltzer P, Mann RM, Iima M, et al. EUSOBI international Breast Diffusion-Weighted Imaging working group. Diffusion-weighted imaging of the breast-a consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur Radiol. 2020 Mar;30(3):1436-1450.
4.Whisenant JG, Romanoff J, Rahbar H, et al. Factors Affecting Image Quality and Lesion Evaluability in Breast Diffusion-weighted MRI: Observations from the ECOG-ACRIN Cancer Research Group Multisite Trial (A6702). J Breast Imaging. 2020 Dec 24;3(1):44-56.
Figures