0146
Detection of Prostate Cancer using Diffusion-Relaxation Correlation Spectrum Imaging with Support Vector Machine Model – A Feasibility Study1Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China, 2MR Collaboration, Central Research Institute, United Imaging Healthcare, Shanghai, China
Synopsis
For clinical prostate examination, magnetic resonance imaging (MRI) is a significant imaging modality. In this study, the feasibility of diffusion-relaxation correlation spectrum imaging (DR-CSI) combined with a support vector machine (SVM) model for detecting PCa in vivo was initially explored, and its diagnostic performance was evaluated and compared with the Prostate Imaging-Reporting and Data System (PI-RADS) score based on multiparametric MRI (MP-MRI). The DR-CSI combined with SVM model may suggest additional clinical value and potential to improve the detection of PCa.
Introduction
Prostate cancer (PCa) is the second most frequently occurring cancer among males, with the highest incidence rates in over 60% countries of the world, and the leading cause of cancer-related death in many countries1. For clinical prostate examination, magnetic resonance imaging (MRI) is a significant imaging modality that provides superb soft tissue contrast and functional evaluation. To date, multiparametric MRI (MP-MRI) has been proven to be a promising noninvasive tool for PCa detection2-4. However, with the increasing applications of MP-MRI with the Prostate Imaging-Reporting and Data System Version 2.1 (PI-RADS v2.1) for PCa diagnosis, it was found that approximately 15-30% of clinically significant cancers were lost5,6. The novel diffusion-relaxation correlation spectrum imaging (DR-CSI) method was proposed. And the machine learning technique, the support vector machine (SVM) model, was introduced to combine with DR-CSI7,8. The purpose of this study was to evaluate the performance of in vivo diffusion-relaxation correlation spectrum imaging (DR-CSI) with support vector machine (SVM) in detecting PCa.Methods
Two hundred forty-seven consecutive patients with elevated PSA ≥7 ng/mL or digital rectal examination positivity underwent prostate MRI between August 2020 and April 2021. Patients were enrolled in three stages. In the exploration stage, patients with PI-RADS ≥ 3 lesions or DRE positivity underwent biopsy, and a PCa detection model utilizing an SVM model was established based on the DR-CSI and biopsy results. The performance of the DR-CSI model was compared with the traditional ADC and T2 values. In the validation stage, an optimal filter scale for the SVM model was chosen with the images used to detect PCa from biopsy, in which the biopsies were carried out on patients with PI-RADS ≥ 3 lesions or positive results according to the PCa detection model or DRE positive. The performance of different mapping methods was compared to choose an appropriate scale. In the test stage, the PCa detection model was used to predict PCa, and the results were compared with PI-RADS scores as well as the gold standard, the biopsy results of patients with the same criteria as in the validation stage. The diagnostic performance of the DR-CSI model and PI-RADS was assessed.Results
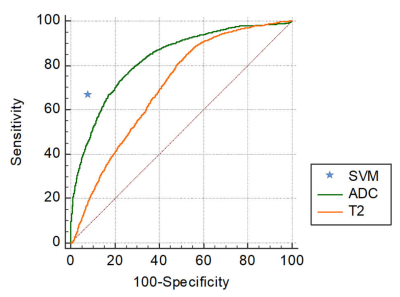
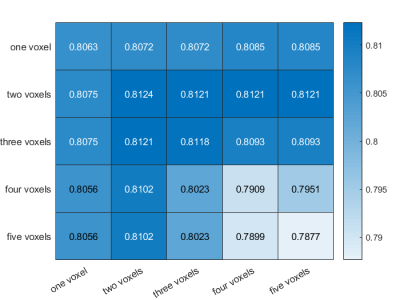
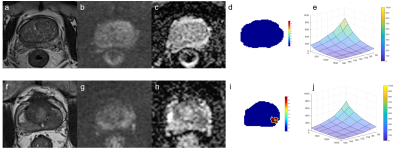
In the exploration stage, the DR-CSI was more accurate than the traditional ADC (0.87 vs. 0.81, p < 0.01) and T2 value (0.87 vs. 0.70, p < 0.01) at the highest Youden index point (Figure 1). In the validation stage, the largest Dice ratios were found with the long axis and short axis having at least 2 and 2 pixels, respectively (Figure 2). In the test stage, considering PI-RADS ≥ 3 and ≥ 4 as the cut-off values, DR-CSI had higher accuracy than PI-RADS (PI-RADS ≥ 3, 71% vs. 40%, p=0.003; PI-RADS ≥ 4, 71% vs. 49%, p=0.031). Considering clinically significant PCa, DR-CSI had higher accuracy than PI-RADS ≥ 3 (58% vs. 36%, p=0.041). However, there was no significant difference in accuracy between DR-CSI and PI-RADS ≥ 4 (58% vs. 58%, p=1.000). Two typical cases are represented in Figure 3.Discussion
The accurate and early diagnosis of PCa based on a noninvasive method holds guiding significance for the choice of the optimal surgical intervention as well as prognosis. In this study, the feasibility of DR-CSI combined with an SVM model for detecting PCa in vivo was initially explored, and its diagnostic performance was evaluated and compared with the PI-RADS score based on MP-MRI. The optimal performance of the former method suggested additional clinical value and potential to improve the detection of PCa, which provided an alternative method for PCa characterization. We also evaluated the performance of the DR-CSI model in detecting cs-PCa. Although the performance of the DR-CSI model did not exceed that of PI-RADS, it still has some practical significance. As the result of the DR-CSI model can be automatically derived with SVM, the method also provides a potential idea to solve the problem of lacking reproducibility with PI-RADS in diagnosis. Meanwhile, as the model was established to differentiate cancer lesions from benign lesions, the model certainly lacks high precision to distinguish between cc-PCa and cs-PCa. The performance in detecting cs-PCa may further improve if only lesions with GS ≥ 7 are used in modelling.Conclusions
The DR-CSI combined with SVM model may improve the diagnostic accuracy of prostate cancer.Acknowledgements
This study was supported by Science and Technology Commission of Shanghai Municipality (Contract grant number: 18DZ1930104) and Shanghai Jiao Tong University medical-engineering cross fund (Contract grant number: YG2021QN27).References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249.
2. Caglic I, Barrett T. Optimising prostate mpMRI: prepare for success. Clin Radiol. 2019;74(11):831-840.
3. Richenberg J, Logager V, Panebianco V, Rouviere O, Villeirs G, Schoots IG. The primacy of multiparametric MRI in men with suspected prostate cancer. Eur Radiol. 2019;29(12):6940-6952.
4. Zawaideh JP, Sala E, Shaida N, et al. Diagnostic accuracy of biparametric versus multiparametric prostate MRI: assessment of contrast benefit in clinical practice. Eur Radiol. 2020;30(7):4039-4049.
5. Sathiadoss P, Schieda N, Haroon M, et al. Utility of Quantitative T2-Mapping Compared to Conventional and Advanced Diffusion Weighted Imaging Techniques for Multiparametric Prostate MRI in Men with Hip Prosthesis. J Magn Reson Imaging. 2021.
6. Kitzing YX, Prando A, Varol C, Karczmar GS, Maclean F, Oto A. Benign Conditions That Mimic Prostate Carcinoma: MR Imaging Features with Histopathologic Correlation. Radiographics. 2016;36(1):162-175.
7. Lee HJ, Hwang SI, Han S-m, et al. Image-based clinical decision support for transrectal ultrasound in the diagnosis of prostate cancer: comparison of multiple logistic regression, artificial neural network, and support vector machine. Eur Radiol. 2010;20(6):1476-1484.
8. Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine Learning for Medical Imaging(1). Radiographics. 2017;37(2):505-515.
Figures