0144
Sensing brain plasticity after stroke using magnetic resonance elastography1INSERM UMRS1148 - Laboratory for Vascular Translational Science, University Paris, Paris, France, 2School of Biomedical Engineering and Imaging Sciences, King’s College London, London, United Kingdom, 3Department of Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany, 4School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
Stroke is the leading cause of death globally. The infarcted area undergoes a deprivation of oxygen and energy, followed by multiple transient events and reorganization processes called ischemic cascade. MRI is the reference tool for accurate diagnosis, volumetric assessments and age determination of the lesion. The aim of the present study was to investigate the time course of changes in tissue biomechanics after an ischemic event in mice using MRE and study the relationship between stroke volume and tissue biomechanics, to explore a potential role of MRE in assessing the post stroke recovery.
Introduction
Stroke is the leading cause of death globally [1]. The majority of the strokes have ischemic origin and they are the results of a thromboembolic occlusion of a cerebral artery or its branches. The infarcted area undergoes a deprivation of oxygen and energy, followed by multiple transient events and reorganization processes called ischemic cascade, involving the formation of reactive oxygen species, release of glutamate, neuronal death, angiogenesis, inflammation [2, 3]. This leads to irreversible tissue damages. Multiparametric magnetic resonance imaging (MRI) is the reference tool for accurate diagnosis, volumetric assessments and age determination of the lesion [4, 5]. However the validity of MRI biomarkers as a measure of outcome has not been established, since it gives no information about the clinical condition of the subject. Magnetic Resonance Elastography (MRE) could offer valuable and complementary information about the viscoelastic properties of the brain tissue, which are inevitably impacted by the events following the onset of a stroke. The aim of the present study was to investigate the time course of changes in tissue biomechanics estimated by MRE after an ischemic event in mice, with unprecedented quality. Moreover we studied the relationship between stroke volume and tissue biomechanics, to explore a potential role of MRE in assessing the post stroke recovery.Methods
Stroke was induced in 5 mice using intra-arterial suture occlusion of the middle cerebral artery (MCAo) [6]. Imaging was performed on a 7T MRI preclinical scanner (Bruker, Ettlingen, Germany; gradient strength 660 mT/m) using an 8.6 cm body coil for transmission and a 2 cm surface coil for reception. The imaging was performed under anaesthesia, induced with isoflurane delivered via a nose cone, monitoring constantly the respiration rate. Shear wave vibrations were induced by using a custom-built bed with the head fixated in a cantilever assembly connected to a linear motor, source of the vibrations. A multi-slice, single spin echo MRE sequence (TR/TE 1600/26 ms; FOV 19.2 mm; matrix 64 × 64; 1 average; 4 wave phases; 35 slices; isotropic resolution 0.250 mm; vibration frequency 700Hz) was used [7]. The MRE data were processed in Fourier Space to remove high frequency noise using a low pass Blackmann Harris filter and were subsequently reconstructed as in Sinkus et al. [8]. This allowed to calculate the complex shear modulus G*=G’ +iG’’, where G’ (Gd) is the shear stiffness and G’’ (Gl) is the shear viscosity, and the phase angle Y=2/π*atan(Gl/Gd) in [0,1] describing whether the material is more sping-like (Y=0) or more viscous-like (Y=1). Brain viscoelastic properties were assessed in the mice prior to the stroke induction and longitudinally 24h and 7 days after the ischemic event.Results and discussion
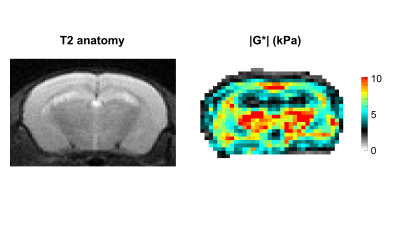
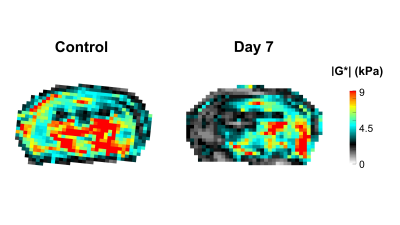
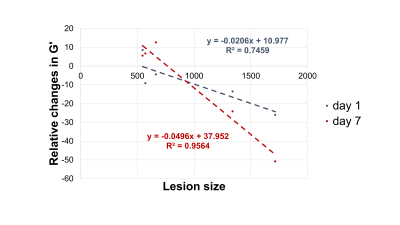
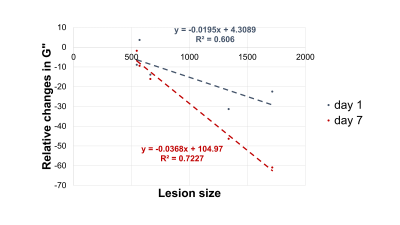
In Figure 1 the stiffness of a control mouse is showed with the corresponding anatomical image, acquired with a T2 MRI scan. The brain biomechanics shows high symmetry between left and right lobe and a relation with the underlying morphological structure. In Figure 2 the brain viscoelastic modulus of mouse before and 7 days after the stroke are showed. Pronounced differences between healthy and infarcted area are visible to the naked eye on the elastogram of the viscoelastic modulus. The infarcted area is characterized by lower values of the viscoelastic modulus, reflecting the disruption of neuronal integrity and of the extracellular matrix, and the necrosis. These findings are in agreement with the literature [2] . Furthermore we evaluated the correlation between the size of the lesions (segmented from MRE magnitude images) and the relative change of G’ (Figure 3) and G” (Figure 4) at day 1 and day 7 after the stroke. The control values for the viscoelastic modulus were taken as reference. G’ and G” relative change correlate with the size of the stroke. Moreover at day 7 the relative change of the biomechanics is substantially higher than the one at day 1, confirming the ongoing plasticity of the brain tissue [9]. This trend shows that biomechanical properties are sensitive to the cascade of tissue degradation and regeneration happening after an ischemic event. In particular smaller lesions show better recovery of the tissue properties with respect to bigger lesion, that show a substantial decrease of the viscoelastic modulus. Previous studies suggested that the size of lesions correlates with recovery outcomes, confirming our findings [10, 11].Conclusions
In this work we investigated the time evolution of brain biomechanics after an ischemic event, using MRE in mice. Our preliminary data show that infarcted tissue is softer than healthy brain tissue, confirming previous studies. Moreover we found a correlation between relative changes in tissue biomechanics and lesion size, as early as at 1 day after stroke. These findings are in line with published clinical studies. However the validity of stroke volume as a measure of outcome has been questioned by many researchers, since it gives no information about the clinical condition of the subject [10]. Therefore we expect MRE to provide further insights in the understanding of the tissue integrity and potentially constitute a relevant measure to assess the outcome of stroke therapy.Acknowledgements
No acknowledgement found.References
1. Lopez, A.D., et al., Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. The lancet, 2006. 367(9524): p. 1747-1757.
2. Freimann, F.B., et al., MR elastography in a murine stroke model reveals correlation of macroscopic viscoelastic properties of the brain with neuronal density. NMR in Biomedicine, 2013. 26(11): p. 1534-1539.
3. Fluri, F., M.K. Schuhmann, and C. Kleinschnitz, Animal models of ischemic stroke and their application in clinical research. Drug design, development and therapy, 2015. 9: p. 3445. 4. Wouters, A., et al., Association between time from stroke onset and fluid-attenuated inversion recovery lesion intensity is modified by status of collateral circulation. Stroke, 2016. 47(4): p. 1018-1022. 5. Kao, Y.-C.J., et al., Role of genetic variation in collateral circulation in the evolution of acute stroke: a multimodal magnetic resonance imaging study. Stroke, 2017. 48(3): p. 754-761.
6. Chiang, T., R.O. Messing, and W.-H. Chou, Mouse model of middle cerebral artery occlusion. Journal of visualized experiments: JoVE, 2011(48).
7. Schregel, K., et al., Characterization of glioblastoma in an orthotopic mouse model with magnetic resonance elastography. NMR in Biomedicine, 2018. 31(10): p. e3840.
8. Sinkus, R., et al., Rheological determinants for simultaneous staging of hepatic fibrosis and inflammation in patients with chronic liver disease. NMR Biomed, 2018. 31(10): p. e3956.
9. Cirillo, C., et al., Post-stroke remodeling processes in animal models and humans. Journal of Cerebral Blood Flow & Metabolism, 2020. 40(1): p. 3-22.
10. Saunders, D.E., A.G. Clifton, and M.M. Brown, Measurement of infarct size using MRI predicts prognosis in middle cerebral artery infarction. Stroke, 1995. 26(12): p. 2272-2276.
11. Beloosesky, Y., et al., The importance of brain infarct size and location in predicting outcome after stroke. Age and ageing, 1995. 24(6): p. 515-518.
Figures