0142
Acute Ischemic Stroke: Infarct Core Estimation from Threshold-based DWI Delineation Depends on the DWI protocol
Jonathan Rafael-Patiño1,2, Elda Fischi-Gomez1, Sebastian Otálora3, Veronica Ravano1,2,4, Guillaume Saliou1, Steven David Hajdu1, Tobias Kober1,2,4, Roland Wiest3, Patrik Michel5, Richard McKinley3, and Jonas Richiardi1
1Radiology Department, Centre Hospitalier Universitaire Vaudois and University of Lausanne., Lausanne, Switzerland, 2LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 3Support Center for Advanced Neuroimaging (SCAN), University Institute of Diagnostic and Interventional Neuroradiology, University of Bern, Inselspital, Bern University Hospital., Bern, Switzerland, 4Advanced Clinical Imaging Technology, Siemens Healthcare AG., Lausanne, Switzerland, 5Stoke Center, Neurology Service, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
1Radiology Department, Centre Hospitalier Universitaire Vaudois and University of Lausanne., Lausanne, Switzerland, 2LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 3Support Center for Advanced Neuroimaging (SCAN), University Institute of Diagnostic and Interventional Neuroradiology, University of Bern, Inselspital, Bern University Hospital., Bern, Switzerland, 4Advanced Clinical Imaging Technology, Siemens Healthcare AG., Lausanne, Switzerland, 5Stoke Center, Neurology Service, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
Synopsis
In acute stroke assessment, reference clinical trials such as DEFUSE-3 use absolute thresholds on ADC maps to define the infarct core. Yet, the variance of ADC values, but not the mean, change with the number of diffusion directions and the specific directions in a 4-directions sampling scheme. The resulting shifts in ADC distribution tails result in an overestimation of the infarct core. This has implications for multi-centric trials, where infarct sizes and locations may be biased simply due to diffusion protocol differences and/or different head positions with respect to B0.
Introduction
Early and accurate diagnosis of ischemic stroke is crucial to identify targets for emergency revascularization treatments and improving long term outcomes. Current guidelines advocate the use of DWI to diagnose acute ischemic stroke and for the perfusion-diffusion weighted imaging (PWI/DWI) mismatch for patients with ischaemic stroke of 6-24 h duration (known onset time[2,3]). Whilst PWI provides an assessment of cerebral hemodynamics, DWI enables estimating the volume of the final infarct when no early reperfusion occurs. The volumetric difference between PWI/DWI (mismatch) is a surrogate for the penumbra: the severely hypoperfused, neurophysiologically silent brain tissue potentially salvageable[3,4]. To shorten the scan time, generally only up to four diffusion gradients are acquired in acute stroke MRI. The infarct core is segmented by thresholding the voxel intensities of the apparent diffusion coefficient image (ADC) extracted from the DWI. It is a fixed threshold ranging from 600-620 x10-6 mm2/s, suggested as optimal by several studies[5,9], but still subject to debate. In this work, we studied the effect of different gradient schemes on the segmentation of the infarcted core based on ADC thresholding. Our direction subsampling study hereby serves as a proxy for the effect of multi-site protocol variability and the effect of direction misalignments due to head tilting during acquisition[9].Methods
Anonymous patients who were treated for an acute ischemic stroke at the University Hospital of Lausanne were included in the analysis if MR imaging was available. MRI was performed on a 3T MRI system (MAGNETOM Vida, Siemens Healthcare, Erlangen, Germany) (see Figure 1 for demographics and scan parameters). T2 images were registered to the non-weighted DW images using FSL-FLIRT and tissue segmented using FSL-FAST. DWI[8] were skull-stripped using Mrtrix[6]. ADC was computed using the mean exponential decay of the diffusion signal[7] (Figure 2C). The multi-centre variability was simulated by subsampling DWI images (from the whole 20-directions dataset) in non-overlapping sets of three orthogonal directions plus an additional one (4-directions scheme) (Figure 2B), mimicking the diffusion acquisition in the hospital ICU unit. Overall, 14 ADC maps were computed for each subject. The infarct core was segmented from the ADC using the 620 mm/s2 intensity threshold; spurious regions (volume <1 ml), were cleaned using morphological operators. We evaluated the impact of gradient direction subsampling on ADC distributions within WM voxels, and on the corresponding infarct volume and location. For scalar quantities (mean ADC, sd ADC, and infarct volume), we fit a negative binomial mixed-effects model with the scalar quantity of interest as the dependent variable, subsampling scheme (20-directions versus 14 variations of 4-directions) as the fixed effect of interest, age, sex, and WM volume as covariates, and a random intercept random effect per subject. Bland-Altmann analysis of the infarct volume was used to assess the agreement between the full directions scheme and the subsampled schemes. Infarct core location consistency was assessed by the Jaccard coefficients (full scheme vs sampled schemes).Results
Results are shown in Figure 3. While the mean ADC remained stable with respect to the direction sampling scheme within the WM, the ADC standard deviation changed indicating a widening of the distribution, with a heavier left tail for the 4-directions scheme (Figure 3). Infarct volumes were overall over-estimated in the 4-direction schemes with a median bias across the 14 repetitions of 1.0 ml (range= 0-3 ml) and a range of agreement between 3.6 and 13.5 ml depending on the specific 4-directions scheme. This effect remained significant when correcting for age, sex and region volume (sampling scheme effect z=-3.3, p=1x10-3). Infarct volume differences were even more pronounced between the 14 realizations of the 4-directions schemes, with a range of agreement between 8.9 and 15.3 ml, and the choice of directions remained significant when correcting for age, sex, and region volume (ANOVA in 4-directions data, comparing model with and model without direction term, X2=38.8, p=2x10-4, delta AICc=11.0). Figure 4 shows, for an exemplary patient, the spatial overlap between infarct core locations computed using different directions. Figure 5 quantifies the spatial overlap using the Jaccard coefficient.Discussion and Conclusions
Our study shows how a different set of orientations (due to protocol differences and/or different head positions with respect to B0) has a significant effect on the distribution’s spread of the ADC in WM. Such changes affect the determination of the infarct core volume and location. For multi-centric trials, infarct sizes and locations may be biased simply due to diffusion protocol differences or patients relative head position. Whilst our work suggests that differences in infarct segmentation can be caused by the sampling of diffusion directions, other possible sources of variability should also be taken into account in multi-sites analysis (eg. slice-thickness, echo time, effective diffusion times and hardware-dependent artifacts). Additionally, even if GM is also affected by ischemic stroke, this work only focuses on WM as the criteria for ADC segmentation used (ADC < 620 s/mm2) is only applicable to WM. Further work will study the effect of the sampling scheme protocol in GM.Acknowledgements
This project was co-financed by lnnosuisse (Innovation Project 43087.1 IP-LS), and received financial support from Siemens Healthcare Switzerland.
References
- Kleindorfer, D. O. et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack; A guideline from the American Heart Association/American Stroke Association. Stroke (2021). doi:10.1161/STR.0000000000000375.
- Berge, E. et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. European Stroke Journal vol. 6 (2021).
- McKinley, R. et al. Fully automated stroke tissue estimation using random forest classifiers (FASTER). J. Cereb. Blood Flow Metab. 37, 2728–2741 (2017).
- Albers GW, et. al. DEFUSE Investigators. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006 Nov;60(5):508-17. doi: 10.1002/ana.20976. PMID: 17066483.
- Straka, et al. Real-time diffusion-perfusion mismatch analysis in acute stroke. J. Magn. Reson. Imaging 32, 1024–1037 (2010).
- Tournier, Jacques-Donald et al. “MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation.” bioRxiv (2019): n. pag.
- Mascalchi, Mario et al. “Diffusion-weighted MR of the brain: methodology and clinical application.” La Radiologia medica vol. 109,3 (2005): 155-97
- M.W. Woolrich, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage, 45:S173-86, 2009
- Pistocchi, et al. MRI software for diffusion-perfusion mismatch analysis may impact on patients’ selection and clinical outcome. Eur Radiol (2021). https://doi.org/10.1007/s00330-021-08211-2
Figures
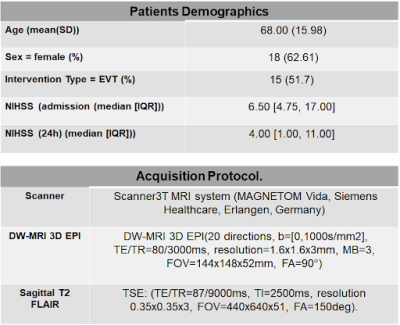
Figure 1: Patients demographics and acquisition protocol information. A total of 29 patients were included in the statistics.
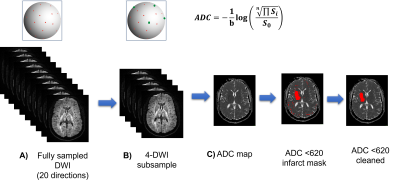
Figure 2: The subsampling procedure simulates a new acquisition protocol with limited acquisition directions. Each subsample consists of a new set of 3 orthogonal directions plus one random direction (B). Each new set can be considered as a new repetition where local orientation w.r.t to the subject changed due to head tilting. From the subsampled DWI, the corresponding ADC map is computed (C). The infarct core mask is computed using a fixed 620x10-9 m2/s threshold. Spurious regions are removed using morphological operations.
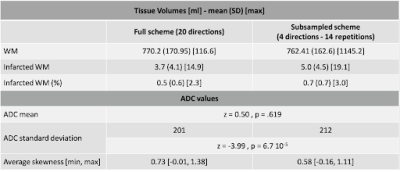
Figure 3. Infarct tissue volume and ADC statistic for both sampling schemes.
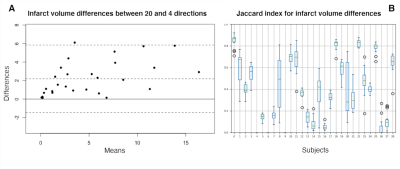
Figure 4: A) Bland-Altman plot of the infarct core volume differences (in ml) between the 20 directions and the 4 directions scheme for all patients. B) Jaccard index plot for each subject between the infarct mask of the 20 directions vs the 14 samples with 4 directions. A Jaccard index of zero indicates no intersection between the infarct masks (due to no infarct core in any of the two subsets). A Jaccard score of one indicates full overlap. Important differences in infarct core locations can be observed across various subsampling schemes.
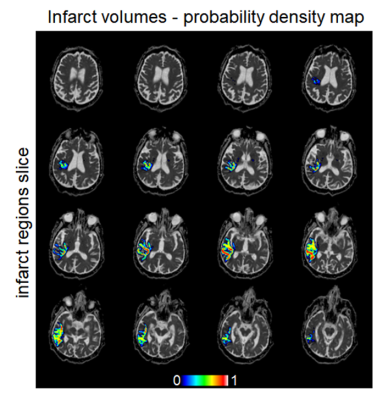
Figure 5: Infarct probability mask computed from the full set of fourteen subsamples for a single subject. For each subsample, the binary infarct mask was computed; all such infarct masks were added together, then divided by the total number of subsampled schemes (14). Warmer colours indicate more consensus for infarct core voxels across the subsampled schemes, while voxels in cooler colours indicate that fewer subsampling schemes would assign them to the infarct core. Considerable variability in location can be observed across subsampled schemes.
DOI: https://doi.org/10.58530/2022/0142