0140
Mechanism of recovery following stem cell therapy in ischemic stroke: cell or secretome?1National High Magnetic Field Laboratory, Florida State University, Tallahassee, FL, United States, 2Chemical & Biomedical Engineering, FAMU-FSU College of Engineering, Tallahassee, FL, United States, 3University of California, Los Angeles, CA, United States, 4Department of Biomedical Sciences, Florida State University, Tallahassee, FL, United States
Synopsis
This study interrogates human mesenchymal stem cell (hMSC) derived treatments (cells versus secretome) in a preclinical transient ischemic stroke model. Biochemical markers of tissue recovery measured longitudinally over 21 d via sodium chemical shift imaging and proton relaxation-enhanced MR spectroscopy demonstrated that the arterial injection of extracellular vesicles produced in vitro by hMSC improved outcomes over ischemic stroke, albeit not to the level of direct cellular implantation. For the first time, novel MRI-compatible labeling provided the initial visualization of biodistribution and estimated permanence of hMSC-derived extracellular vesicles in the ischemic brain.
Introduction
Bone marrow derived human mesenchymal stem cells (hMSC) have been assessed for their therapeutic efficacy in a range of pathologies, including cancer, Alzheimer’s disease and stroke. Trophic and immunomodulatory effects, as well as their role in cell-to-cell communication, support hMSC as promising candidates for cell therapy.1 However, direct hMSC implantation compared to the impact of their secretions alone on therapeutic outcome, either locally at the lesion or by recruiting regenerative progenitor cells, has remained elusive. Of particular interest for potential therapeutics are extracellular vesicles (EV), which--as messengers of the hMSC secrotome--carry immunomodulatory and regulatory secretions that impact synaptic transmission, neuronal differentiation, angiogenesis and neuronal projection.2 Similar to cellular therapy, in vivo tracking of EV is imperative to determining biodistribution. A novel EV labeling method with ultra-small superparamagnetic iron oxide (USPIO) contrast was used here to confirmed localization to the ischemic region. Tissue recovery metrics were assessed via ultra-high field (21.1 T) sodium (23Na) chemical shift imaging (CSI) and 1H relaxation-enhanced MR spectroscopy (RE-MRS), providing insight into ionic and metabolic homeostasis in response to the delivery of hMSC versus their derived EV in a cerebral ischemic stroke model.Methods
Cell Culture and EV Processing: hMSC acquired from Tulane Center for Stem Cell Research and Regenerative Medicine were characterized based on plastic adherence and surface markers.3 EV were isolated and purified from hMSC following one week of hypoxic preconditioning; EV underwent robust characterization to ensure purity.4 Following EV harvest, hypoxic hMSC were exposed to 7.47-µg Fe/mL micron-sized particles of iron oxide (MPIO, Bangs Laboratory, Inc.) prior to injection. To accommodate the smaller size, EV underwent labeling with 0.5-mg/mL ultra-small super-paramagnetic iron oxide (USPIO, Sigma Aldrich) via sonication and purified utilizing a novel polyethylene glycol (PEG) enrichment method outlined in Figure 1. Both therapies were re-suspended in 50-μL saline for in vivo injection.Animal Model: A transient middle cerebral artery occlusion model5 was instituted in male Sprague-Dawley rats (200-250 g) for 1 h to induce striatal ischemia, followed by immediate arterial injection of hMSC-derived therapy (cells or EV) or saline only. Neuromotor assessment was conducted concurrently with MR scanning to 21-d post-ischemia.
MR Acquisitions: Data were acquired using the 21.1-T, 900-MHz vertical magnet at the NHMFL equipped with a linear birdcage dual-tuned 23Na/1H radio frequency coil. Therapy administration was confirmed for both EV (1-4 h) and hMSC (1 d) with gradient recalled echo images (50x50-µm in-plane resolution). 23Na 3D CSI was acquired at 1-mm isotropic resolution. RE-MRS evaluated metabolites in both ischemic and contralateral hemispheres. Selective bandwidth excitation pulses targeted lactate, creatine, choline and N-acetyl aspartate, while avoiding water, with spatial selectivity imparted by adiabatic selective refocusing (LASER) pulses.6 T2-weighted images provided anatomical reference to the ischemic lesion and contralateral alignment.
Analysis: EV biodistribution and clearance were assessed in ParaVision 5.0 (Bruker Biospin, Inc). CSI data reconstructed in MATLAB were zero-filled to 0.5-mm isotropic resolution for volumetric and signal analysis in Amira 3D Visualization Software (Thermo Fisher Scientific, Inc). A signal threshold generated from the contralateral hemisphere was used to define the ischemic lesion. Peaks for RE-MRS data were assigned according to previous literature (NAA 2.0 ppm, Lac 1.31 ppm, Cre 3.0 ppm, Cho 3.2 ppm), referencing water at 4.7 ppm.7 Metabolite ratios to choline were used to track metabolic levels longitudinally. Statistical significance was calculated according to mixed-model effects with p < 0.05, and all graphs are mean ± SD.
Results
MRI confirmed presence of USPIO-labeled EV in the ischemic region immediately following MCAO and therapy administration, with complete clearance by 24 h as expected (Figure 2). hMSC remained in cerebral tissue with total clearance by 7 d.23Na 3D CSI demonstrated that hMSC- and EV-injected animals exhibited significant ischemic lesion reduction by 7 d (Figure 3). In contrast, control ischemic animals first exhibited significantly elevated lesion volume on day 3 followed by reduction only at 21 d. As demonstrated by 23Na signal reductions, both hMSC and EV evoked therapeutic response, illustrated by maintenance of significantly lower sodium levels in both the ischemic and contralateral hemispheres compared to control animals. Furthermore, reduction in lactate levels closely paralleled improved sodium response in hMSC and EV groups (Figure 4). Functional recovery assessed via several neuromotor tests as well as weight (Figure 5) reinforce the therapeutic efficacy generated by hMSC-derived EV.
Discussion
For the first time, USPIO labeling of EV generated contrast in cerebral tissue immediately after administration, following a novel sonication and PEG-based enrichment protocol. EV did not exhibit changes in size distribution profile following this labeling procedure. Previous studies have demonstrated 23Na CSI and 1H RE-MRS are highly sensitive biomarkers of therapy efficacy and have been able to differentiate successful from ineffective therapies as early as 1 d post-injection, hence are suitable metrics to be used here to determine therapeutic efficacy of EV. Ischemic lesion volume and signal changes from 23Na CSI as well as metabolic alterations show that hypoxic hMSC-derived EV evoke a therapeutic response similar though perhaps more muted than their cellular counterparts.Conclusion
Efficacy of hMSC-derived extracellular vesicles labeled with USPIO localize to the ischemic legion and evoke a therapeutic response after a single bolus injection.Acknowledgements
All work has been conducted in accordance with the Florida State University Animal Care and Use Committee. This work was supported by the US NIH (F31-NS115490 awarded to S.H. and R01-NS102395 awarded to S.C.G.). The National High Magnetic Field Laboratory is funded by the US NSF (DMR-1644779) and the State of Florida.
References
1. Doeppner, T. R. et al. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Post-ischemic Immunosuppression. Stem Cells Transl. Med. 4, 1131–43 (2015).
2. Otero-Ortega, L. et al. Role of Exosomes as a Treatment and Potential Biomarker for Stroke. Translational Stroke Research 10, 241–249 (2019).
3. Yuan, X., Rosenberg, J. T., Liu, Y., Grant, S. C. & Ma, T. Aggregation Preconditioning Enhances Survival and Efficacy of Human Mesenchymal Stem Cells in Stroke Treatment. Cytotherapy 21, 1033–1048 (2019).
4. Rider, M. A., Hurwitz, S. N. & Meckes, D. G. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci. Rep. 6, 23978 (2016).
5. Longa, E. Z., Weinstein, P. R., Carlson, S. & Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20, 84–91 (1989).
6. Shemesh, N., Rosenberg, J.T., Dumez, J.N., Muniz, J.A., Grant, S.C., Frydman, L. Metabolic properties in stroked rats revealed by relaxation-enhanced magnetic resonance spectroscopy at ultrahigh fields. Nat. Commun. 5, (2014).
7. Govindaraju, V., Young, K. & Maudsley, A. A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 13, 129–153 (2000).
Figures
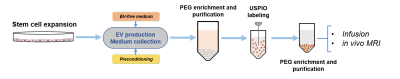
Figure 1. Schematic of novel USPIO EV labeling method. EV were harvested from hMSC media as described previously using polyethylene glycol (PEG) enrichment.4 An additional PEG enrichment step following EV sonication with label enabled purification of USPIO-labeled EV for in vivo administration.
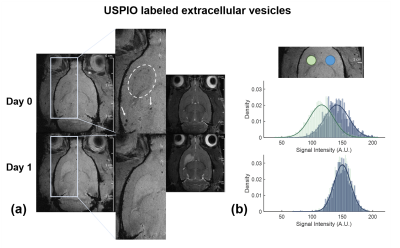
Figure 2. Successful localization of EV in ischemic region. a) EV visualization in cerebral tissue immediately following MCAO and 1-d post-MCAO in the striatum (white circle) with minimal aggregation (arrows); and b) signal intensity histograms demonstrating substantial contrast within the ischemic lesion (green) compared to contralateral striatum (blue) on day 0, with complete clearance of USPIO contrast at the ischemic lesion by day 1.
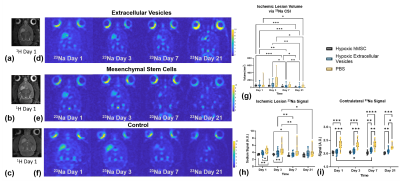
Figure 3. Sodium demonstrates lesion recovery in hMSC and EV administered animals. Representative (a-c) T2W images 1-d and (d-f) 23Na CSI over the entire time course for animals administered EV, hMSC and saline, respectively. Bar graphs demonstrate g) ischemic lesion volume recovery, h) ischemic lesion 23Na signal and i) contralateral hemisphere 23Na signal. Statistical significance calculated using a mixed-model effects with Tukey’s multiple comparison post-hoc test. Significance indicated by *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. All values presented as mean ± SD.
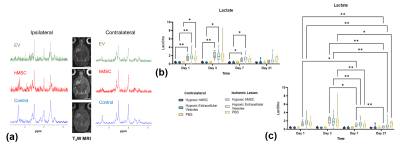
Figure 4. Energetics measured via 1H RE-MRS. a) Representative RE-MRS 1-d post-MCAO for all three groups. Lactate levels indicate return of energetics for hMSC and EV groups b) between hemispheres and c) longitudinally. Statistical significance calculated using a mixed-model effects with Tukey’s multiple comparison post-hoc test. Significance is indicated by * p < 0.05 and ** p < 0.01. All values presented as mean ± SD.
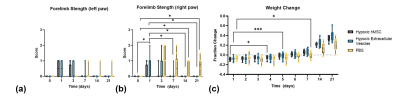
Figure 5. Functional recovery and weight change support MR findings. Bar graphs represent strength test for a) left and b) right forelimbs as well as c) change in weight over 21 d. Statistical significance calculated using a mixed-model effects with Tukey’s multiple comparison post-hoc test. Significance is indicated by * p < 0.05 and *** p < 0.001. All values presented as mean ± SD.