0124
GraspCEST: Fast Free-Breathing 3D Steady-State CEST MRI Using Golden-Angle Radial Sparse MRI1Department of Radiology and BioMedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Center for Advanced Imaging Innovation and Research (CAI2R) and Department of Radiology, New York University School of Medicine, New York, NY, United States
Synopsis
In this project, we proposed a fast 3D CEST MRI technique called GraspCEST, which is based on the combination of CEST-prepared stack-of-stars golden-angle radial sequence and multicoil compressed sensing reconstruction. Using this technique, we were able to perform lipid suppressed, free-breathing 3D CEST imaging of the liver. We have demonstrated that it is possible to accelerate imaging speed and achieve full Z-spectra acquisition of the liver within 5 min. The GraspCEST technique can be a useful tool in studying glycoNOE in the liver.
Introduction
Chemical exchange saturation transfer (CEST) MRI is a molecular imaging technique that allows for detection of proteins and metabolites through their exchange properties with water. These magnetization transfer mechanisms include direct proton exchange and exchange relayed nuclear Overhauser effect (NOE). Most CEST/NOE experiments require the water signal to be acquired at a few saturation frequency offsets. Therefore, scans can be time-consuming and prone to motion artifacts. For this reason, CEST MRI in the body has been technically challenging. An earlier study testing the feasibility of liver CEST imaging acquired only a single slice and required multiple breath-holds1, which potentially results in slice inconsistencies between different time points. Recently, it has been shown that glycogen in the liver can be measured based on exchange mediated NOE (glycoNOE)2. However the technique is currently limited to animal studies with a single-slice acquisition.The purpose of this project was to develop a fast 3D CEST MRI technique called GraspCEST, which is based on the combination of CEST-prepared stack-of-stars golden-angle radial sequence and multicoil compressed sensing reconstruction3. Furthermore, multi-echo acquisition has been incorporated4, enabling CEST quantification in water-only images without interrupting the steady state during data acquisition. The technique has been tested in phantom and free-breathing liver imaging experiments.
Methods
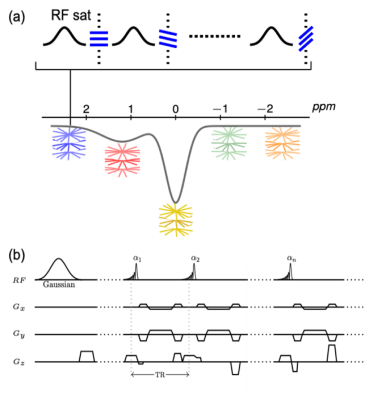
Sequence and Reconstruction: A CEST-prepared sequence with stack-of-stars golden-angle radial trajectory has been implemented, as shown in Figure 1. CEST preparation is performed right before the acquisiton of each radial stack (i.e., all spokes corresponding to one acquisiton angle). There is no delay between acquisitions of different stacks to achieve steady-state CEST imaging. Acquisition of each radial stack is done with centric-out ordering, and the rotation from one stack to the next follows the golden-angle scheme (i.e., adding 111.25°). GRASP reconstruction was performed to reconstruct dynamic images from the data by exploiting spatiotemporal image correlations.Imaging Experiments: For CEST labeling, each saturation pulse was applied with a duration of 50 ms and a power of 0.7 μT, followed by a short spoiling period (10 μT/m, 2 ms) and acquisition of one radial stack (16 partitions in this study). All imaging experiments were performed on a 3T clinical MRI scanner (Skyra, Siemens Healthcare, Erlangen, Germany).
Parameters of phantom experiments: 16 slices, FOV = 192x192 mm2, in-plane resolution = 2x2 mm2, slice thickness = 5 mm, TR = 2.43 ms, TE = 1.12 ms, and FA = 5°. 45 dynamic measurements were collected at 390 ppm as reference and within a frequency range of -4.7 to 4.7 ppm. 150 radial stacks were acquired in each dynamic measurement, corresponding to a total scan time of 10.5 min.
Parameters of multi-echo liver experiments: 16 slices, FOV = 350x350 mm2, in-plane resolution 2.7x2.7 mm2, slice thickness = 5 mm, TR = 5.77 ms, TE1 = 1.41 ms, TE2 = 3.01 ms, TE3= 4.61 ms, and FA= 5°. 29 dynamic measurements were collected at 390 ppm as reference and within a frequency range of -7.8 to 7.8 ppm. 200 radial stacks were acquired in each dynamic measurement, corresponding to a total scan time of 14 min.
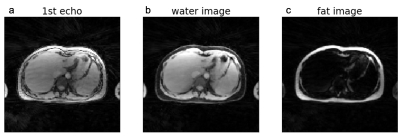
Non-uniform fast Fourier transform (NUFFT) was first performed to reconstruct dynamic images using all acquired spokes. GRASP reconstruction was subsequently performed to reconstruct dynamic images using only a subset of stacks acquired in each measurement (43 stacks for phantom imaging, and 70 for liver imaging), corresponding to ~3 minutes and ~5 minutes scan time, respectively. For the multi-echo liver scan, fat/water separation was performed after image reconstruction.
Results and Discussion
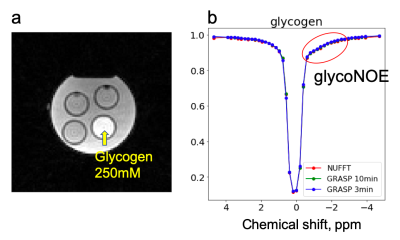
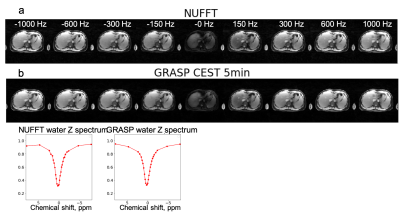
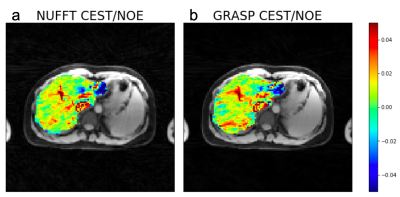
Figure 2 shows results from the glycogen phantom. The glycoNOE effect can be observed between -0.5 and -2 ppm. With GRASP, the number of views needed for image reconstruction can be reduced significantly without affecting the resulting Z-spectrum. This allows shortening the experiment from 10 min to 3 min, corresponding to 4 s per frequency offset per 3D volume. Figure 3 shows in-vivo liver images acquired using the multi-echo GraspCEST method, which allows separation of water and fat using the Dixon technique. Throughout the dynamic acquisition, shown in Figure 4, no motion-related artifact was observed. The GRASP reconstruction allows reducing the scan to 5 min while maintaining image quality. Compared to the glycogen phantom, the in-vivo Z-spectra show a broader resonance between -0.5 to -4 ppm, which represents glycoNOE (-0.5 to -2 ppm) and NOE exchange pathways from other mobile proteins (-2 to -5 ppm). GlycoNOE-weighted images were generated by taking the average difference between the acquired water Z-spectra and the Lorentzian fitted5 spectra at -1 to -1.5ppm (Figure 5). It can be seen that the accelerated glycoNOE map obtained with GraspCEST preserves the features of the original map.Conclusion
We present a new 3D steady-state CEST imaging method based on the GRASP technique. The improved motion robustness from radial sampling allows for free-breathing 3D CEST imaging of the liver. Moreover, the compressed-sensing reconstruction can accelerate imaging speed which allowing full Z-spectra acquisition of the liver within 5 min. In both phantoms and in vivo, we demonstrate that the GraspCEST technique can be used to detect the glycoNOE. Further validation and quantification of glycoNOE in vivo is needed.Acknowledgements
This project is supported by NIH R00EB026312.References
1. Chen, S. Z.; Yuan, J.; Deng, M.; Wei, J.; Zhou, J.; Wang, Y. X. Chemical exchange saturation transfer (CEST) MR technique for in-vivo liver imaging at 3.0 tesla. Eur Radiol 2016, 26, 1792-1800.
2. Zhou, Y.; van Zijl, P. C. M.; Xu, X.; Xu, J.; Li, Y.; Chen, L.; Yadav, N. N. Magnetic resonance imaging of glycogen using its magnetic coupling with water. Proceedings of the National Academy of Sciences2020, 117, 3144-3149.
3. Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. “Golden-Angle Radial Sparse Parallel MRI: Combination of Compressed Sensing, Parallel Imaging, and Golden-Angle Radial Sampling for Fast and Flexible Dynamic Volumetric MRI” Magn Reson Med. 2014 Sep;72(3):707-17
4. Benkert T, Feng L, Sodickson DK, Chandarana H, Block, KT. “Free-breathing Volumetric Fat/Water Separation by Combining Radial Sampling, Compressed Sensing, and Parallel Imaging” Magn Reson Med. 2017 Aug;78(2):565-576
5. Jones, C. K.; Huang, A.; Xu, J.; Edden, R. A. E.; Schär, M.; Hua, J.; Oskolkov, N.; Zacà, D.; Zhou, J.; McMahon, M. T.; Pillai, J. J.; van Zijl, P. C. M. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. NeuroImage 2013, 77, 114-124.
Figures