0121
Self-Navigated Radial Free-Breathing Magnetic Resonance Elastography of the Liver with Rapid Motion Encoding in Children at 3T1Radiological Sciences, University of California, Los Angeles, Los Angeles, CA, United States, 2Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States, 3MR Collaborations, Siemens Medical Solutions, Inc., Salt Lake City, UT, United States, 4Pediatrics, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
Hepatic stiffness measured by magnetic resonance elastography (MRE) is a biomarker for hepatic fibrosis. Conventional Cartesian gradient-echo MRE requires breath-holding (BH), which can be challenging for children. While radial free-breathing (FB) MRE overcomes the challenge of BH, the scan time is relatively longer (163 seconds/slice). This study’s objective was to reduce radial FB-MRE scan time by a factor of two with rapid motion encoding (81 seconds/slice) and to improve robustness by performing self-navigated motion compensation. Compared to Cartesian BH-MRE in children at 3T, the proposed rapid radial FB-MRE technique quantified hepatic stiffness with close agreement and similar repeatability.
Introduction
Hepatic stiffness measured by magnetic resonance elastography (MRE) correlates with histopathological staging of hepatic fibrosis 1-4. Conventional 2D Cartesian gradient-echo (GRE) based MRE requires breath-holding (BH) to avoid motion artifacts, which can be challenging, especially in children 5. Previous work showed that 2D radial GRE free-breathing (FB) MRE could overcome the limitations of BH due to its relative insensitivity to motion 6,7. However, these radial GRE FB-MRE methods required longer scans of 2-3 minutes/slice. In this pilot study, we developed a faster radial GRE FB-MRE technique using rapid motion encoding (i.e., rapid GRE MRE encoding) to cut the scan time in half 8 when compared to previous radial FB-MRE techniques 6,7. Furthermore, we investigated self-navigation to compensate for potential variations in breathing motion during the FB scan. We assessed the accuracy and the repeatability of the “rapid radial FB-MRE” technique with and without self-navigation, in comparison with a corresponding rapid fractional Cartesian GRE BH-MRE method (“rapid fractional BH-MRE”) 8,9 in children at 3T.Methods
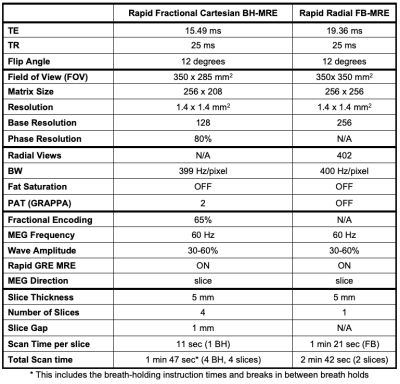
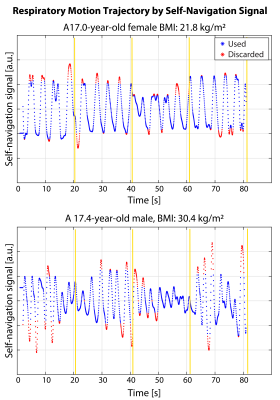
Study Cohort and MRE Experiments: Seven children were enrolled in this IRB-approved HIPAA-compliant study. For age, median$$$\pm$$$interquartile range (IQR) was 15.6$$$\pm$$$3.6; for body-mass index (BMI) median$$$\pm$$$IQR was 50.0$$$\pm$$$57.5 percentile. To assess repeatability, rapid fractional BH-MRE and prototype rapid radial FB-MRE sequences were each acquired twice in the same exam on a 3T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). The imaging parameters were matched as much as possible (Table 1). Rapid radial FB-MRE is a single-slice acquisition (81 sec/slice). Due to time constraints, only 2 matched slices 10 were acquired with rapid radial FB-MRE. To match the slice positions, rapid fractional BH-MRE was scanned before rapid radial FB-MRE during the first scan. The order of the two sequences was randomized for the second scan.MRE Reconstruction: Rapid radial FB-MRE samples the center of k-space every TR. The center of k-space signal from every other TR was used to form a self-navigation waveform representing respiratory motion (Fig. 1). The rapid radial FB-MRE scans were reconstructed (1) without self-navigation and (2) using self-navigation with an 80% acceptance rate to exclude views during larger excursions in respiration. All MRE images were reconstructed using the scanner software.
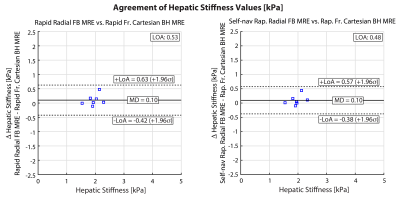
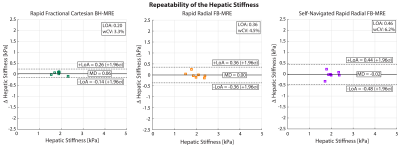
Analysis: Two matched slices from all subjects were used for analyses. Using custom software (MATLAB, Mathworks, MA, USA), hepatic stiffness was measured in regions of interest (ROIs) inside the liver with ≥90% confidence interval (CI), as this was found to be reliable for analysis in children 5. Agreement of the hepatic stiffness measurements was assessed for scan 2 using Bland-Altman (BA) analysis in terms of the mean difference (MD) and 95% limits of agreements (LoA) 11,12. To evaluate the repeatability, BA analysis with MD and LoA, as well as the within-subject coefficient of variation (wCV) were used; wCV was recommended by the Quantitative Imaging Biomarkers Alliance (QIBA) profile for MRE of the Liver (MREL) 13.
Results
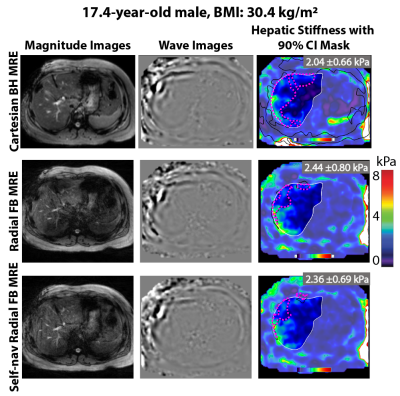
The self-navigation signal amplitude was plotted in Fig. 1 for a 17-year-old female and a 17-year-old male. The MRE images and maps are shown in Fig. 2 for the same 17-year-old male subject. Rapid fractional BH-MRE, rapid radial FB-MRE, and self-navigated rapid radial FB-MRE yielded similar hepatic stiffness measurements for the chosen representative slice. The BA plots in Fig. 3 showed agreement between rapid fractional BH-MRE versus rapid radial FB-MRE, and rapid fractional BH-MRE versus self-navigated rapid radial FB-MRE. The BA plots in Fig. 4 summarized the repeatability for rapid fractional BH-MRE and rapid radial FB-MRE techniques. The wCV for rapid fractional BH-MRE, rapid radial FB-MRE, and self-navigated rapid radial FB-MRE were 3.3%, 4.5%, 6.2%, respectively.Discussion
Previous studies investigated radial FB-MRE in adults 7 and children 6. However, these previous radial FB-MRE methods had a relatively long scan time (163 seconds/slice), which may be suboptimal for clinical protocols. Here, we implemented rapid motion encoded radial GRE FB-MRE in children to reduce the scan time by two-fold (81 seconds/slice) and used self-navigated motion compensation to exclude outlier radial views due to variations in breathing. Both rapid radial FB-MRE techniques achieved good agreement with rapid fractional BH-MRE. Furthermore, self-navigation achieved tighter LoA. All techniques successfully achieved wCV<7%, indicating acceptable repeatability according to QIBA 13,14.Our study had limitations. First, the sample size was small, so we were not able to cover a wide range of hepatic stiffness. Second, the prototype rapid radial FB-MRE sequence is currently a single-slice acquisition. Future work could incorporate simultaneous-multi-slice imaging 15,16. Third, the rapid radial FB-MRE used in this study did not use fractional encoding to decrease TE 9. Lastly, the self-navigation acceptance threshold was set at 80% to limit the undersampling artifacts. Future work could investigate smaller thresholds for a mildly oversampled acquisition, which could improve quantitative accuracy 17.
Conclusion
In this preliminary study, radial FB-MRE with rapid motion encoding reduced FB scan time by a factor of two and was repeatable and in agreement with rapid fractional Cartesian BH-MRE in children at 3T. Rapid radial FB-MRE with self-navigation is a promising technique to measure hepatic stiffness in children when breath-holding is challenging or impractical.Acknowledgements
Research reported in this publication was supported by an Exploratory Research Grant from the UCLA Department of Radiological Sciences, the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK124417, and the National Center for Advancing Translational Sciences under Award Number UL1TR001881. The authors thank Grace Hye Min Kim, Tammy Lambert Graham, Arineh Aghakhani, and the MRI technologists at UCLA.References
1. Idilman IS, Li J, Yin M, Venkatesh SK. MR elastography of liver: current status and future perspectives. Abdom Radiol 2020:1-19.
2. Batheja M, Vargas H, Silva AM, et al. Magnetic resonance elastography (MRE) in assessing hepatic fibrosis: performance in a cohort of patients with histological data. Abdom Imaging 2015;40(4):760-765.
3. Xanthakos SA, Podberesky DJ, Serai SD, et al. Use of Magnetic Resonance Elastography to Assess Hepatic Fibrosis in Children with Chronic Liver Disease. J Pediatr-Us 2014;164(1):186-188.
4. Singh S, Venkatesh S, Wang Z, et al. Diagnostic Performance of Magnetic Resonance Elastography for the Staging of Liver Fibrosis: A Systematic Review and Collaborative Individual Participant Data Meta-Analysis. Am J Gastroenterol 2014;109:S144-S144.
5. Schwimmer JB, Behling C, Angeles JE, et al. Magnetic Resonance Elastography Measured Shear Stiffness as a Biomarker of Fibrosis in Pediatric Nonalcoholic Fatty Liver Disease. Hepatology 2017;66(5):1474-1485.
6. Kafali SG, Armstrong T, Shih S-F, et al. Assessment of Free-Breathing Radial Magnetic Resonance Elastography in Healthy Children and Children with Liver Disease at 3T. 28th Annual Meeting of International Society of Magnetic Resonance in Medicine; 2020. p. Program Number 0087.
7. Holtrop JL, Kannengiesser S, Loeffler RB, Song R, Hillenbrand CM. Free Breathing Radial Magnetic Resonance Elastography. 27th Annual Meeting of International Society of Magnetic Resonance in Medicine. Montreal, Canada; 2019. p. Program Number 4482.
8. Chamarthi SK, Raterman B, Mazumder R, et al. Rapid acquisition technique for MR elastography of the liver. Magn Reson Imaging 2014;32(6):679-683.
9. Rump J, Klatt D, Braun J, Warmuth C, Sack I. Fractional encoding of harmonic motions in MR elastography. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 2007;57(2):388-395.
10. Felker ER, Choi KS, Sung K, et al. Liver MR Elastography at 3 T: Agreement Across Pulse Sequences and Effect of Liver R2*on Image Quality. Am J Roentgenol 2018;211(3):588-594.
11. Obuchowski NA, Reeves AP, Huang EP, et al. Quantitative imaging biomarkers: a review of statistical methods for computer algorithm comparisons. Statistical methods in medical research 2015;24(1):68-106.
12. Raunig DL, McShane LM, Pennello G, et al. Quantitative imaging biomarkers: a review of statistical methods for technical performance assessment. Statistical methods in medical research 2015;24(1):27-67.
13. Committee QMB. MR Elastography of the Liver, Quantitative Imaging Biomarkers Alliance. Profile Stage: Consensus. June 6, 2019.
14. Serai SD, Obuchowski NA, Venkatesh SK, et al. Repeatability of MR elastography of liver: a meta-analysis. Radiology 2017;285(1):92-100.
15. Adluru G, Bieging E, Chen L, Kim D, Wilson BD, DiBella EV. Radial simultaneous multi slice imaging for rapid cardiac imaging. Journal of Cardiovascular Magnetic Resonance 2016;18(S1):O111.
16. Yutzy SR, Seiberlich N, Duerk JL, Griswold MA. Improvements in Multislice Parallel Imaging Using Radial CAIPIRINHA. Magnetic Resonance in Medicine 2011;65(6):1630-1637.
17. Zhong X, Hu HH, Armstrong T, et al. Free‐Breathing Volumetric Liver and Proton Density Fat Fraction Quantification in Pediatric Patients Using Stack‐of‐Radial MRI With Self‐Gating Motion Compensation. Journal of Magnetic Resonance Imaging 2020.
Figures