0117
Acute Effects of High-Intensity Exercise on Brain Mechanical Properties and Cognitive Function1Biomedical Engineering, University of Delaware, Newark, DE, United States, 2University of Delaware, Newark, DE, United States, 3University of Bath, Bath, United Kingdom, 4University of Illinois at Urbana-Champaign, Champaign, IL, United States
Synopsis
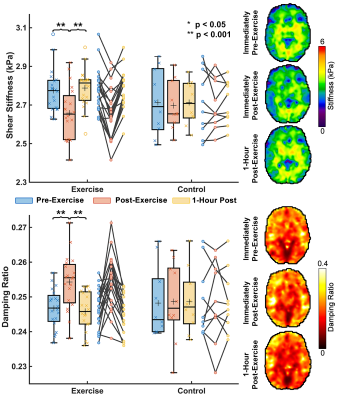
Here we use magnetic resonance elastography (MRE) to quantify brain mechanical properties of 30 individuals aged 19-30 immediately before a high intensity interval training (HIIT) exercise, immediately after exercise, and 1 hour after exercise. We additionally tested subjects’ cognitive performance at these time points through a memory task and two executive function tasks. We found that whole brain stiffness decreased in nearly all subjects immediately after exercise by an average of 4.2% (p<0.0001) and that whole brain damping ratio increased by an average of 3.1% (p=0.001). Performance on all three cognitive tasks improved immediately after exercise (p<0.05).
Introduction
Regular engagement in exercise has profound positive effects on brain health and function, and an increasing body of literature shows that even acute exercise temporarily improves cognitive performance1. However, the neural mechanisms by which acute exercise improves cognition are still largely unknown. One metric by which to characterize brain tissue is through mechanical properties, which can be measured using magnetic resonance elastography (MRE)2. MRE is a phase-contrast MRI technique which uses external vibration and motion encoding gradients to capture tissue displacement, which can then be converted into maps of brain mechanical properties. Mechanical properties are sensitive to subtle changes in brain performance and health and have been seen to reflect differences in cognitive performance3. MRE measures have also reflected brain changes which arise from multiweek exercise interventions4. Understanding the acute neural changes which arise in response to exercise may enlighten the mediative effect between acute exercise and improved cognitive performance.Methods
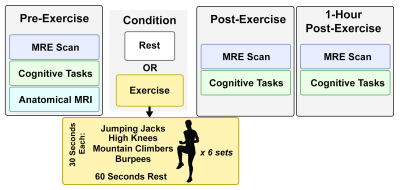
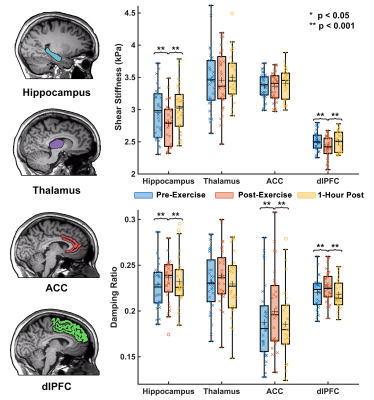
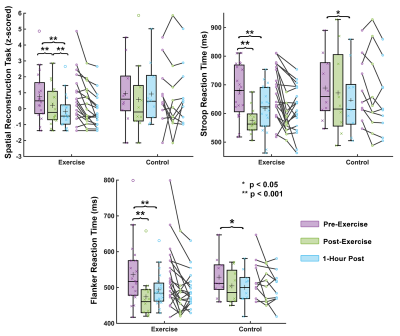
Thirty subjects (15 male, 15 female) ages 19-30 were split into two groups, exercise (N=20) and control (N=10). The exercise group performed an 18-minute high-intensity interval training (HIIT) workout; the control group rested for the same duration. Both groups had an MRI scan and cognitive assessments immediately before, immediately after, and 1-hour after the activity period (Figure 1). Each of the three MRI scans comprised a high resolution OSCILLATE MRE scan5 with 50 Hz vibration, 1.5x1.5x1.5 mm3 resolution, and 80 slices, and a spin-echo field map with matching field-of-view and resolution. OSCILLATE is a multiband spiral MRE sequence6 which uses low-rank image reconstruction to allow for data under-sampling and aqusition acceleration. Each MRE scan took 4 minutes 29 seconds. Additionally, during the first MRI, each subject had an MPRAGE scan at 0.9x0.9x0.9 mm3 resolution for segmentation using Freesurfer, with regions of interest (ROIs) chosen based on known relationships with the cognitive tasks. All MRE displacement data was processed through a nonlinear inversion algorithm (NLI)7 to solve for the complex shear modulus, G, which was converted to viscoelastic shear stiffness, μ=2|G|2/(G’+|G|) and damping ratio ξ=G’’/2G’8. At each of the three assessment points (before, after, and 1-hour), subjects also completed three computerized cognitive tasks: a spatial reconstruction (SR) task to measure relational memory, and the Erikson Flanker and Stoop Tasks to measure executive control. Performance on the SR task depends on the hippocampus, a prominent memory structure, the Flanker task relies on the anterior cingulate cortex (ACC), a structure that supports decision-making, and the Stroop task relies on the ACC and the dorsolateral prefrontal cortex (dlPFC), which is implicated in response inhibition. Finally, the thalamus is a central relay system known to be pivotal in executive functioning.Results
Compared to before exercise, whole-brain stiffness decreased by an average of 4.2% (p<0.0001) immediately after exercise, with nearly all subjects showing some decrease in stiffness. Similarly, immediately following exercise whole-brain damping ratio increased 3.1% on average (p=0.001) (Figure 2). Regional properties were differentially affected by exercise. The hippocampus decreased in µ by 8.5% and increased in ξ by 5.0% following exercise (p<0.05, for both measures), while the thalamus µ and ξ did not significantly change with exercise. Interestingly, the cortex regions responded differently to exercise in the magnitudes of change of µ and ξ, with the dlPFC µ decreasing 4.7% (p=0.0001) while the ACC µ did not significantly change (p=0.147), though both the dlPFC and the ACC exhibited increases in ξ of 4.2% and 7.2%, respectively (Figure 3). In all ROIs, mechanical properties returned to baseline 1-hour after exercise. No significant changes were found in brain mechanical properties in the control group in any ROI across time points. Significant improvements were also found immediately post-exercise for all three of the cognitive tasks. Errors in the SR task were reduced by 19.5% (p=0.002) and response time was lower in the executive functioning tasks (Stroop: 14.2%, p=0.001; Flanker: 11.6%, p=0.003) (Figure 4).Discussion and Conclusion
Acute exercise resulted in both a significant response in brain mechanical properties and a significant improvement in cognitive performance. Exercise was associated with decreased brain stiffness and increased brain damping ratio. This finding comes despite nearly all brain MRE literature showing that greater stiffness is typically associated with greater brain integrity and improved functional performance9. Typically, brain stiffness is driven by the degree of myelination, integrity of the glial matrix, and density of neurons. However, it is likely that in this case, an alternate mechanism is driving the decreases in brain stiffness seen immediately after exercise. One likely option is the acute cerebral hemodynamic response, which occurs from high intensity exercise and involves increased blood flow and vessel diameter, leads to a decreased vascular resistance and increased blood viscosity10. Previous studies have shown that these changes promote improved cognitive performance11 by carrying additional oxygenated blood to the brain tissue, and this hemodynamic response may also be a mechanism by which measurable mechanical properties are changing. In the future these experiments will be replicated in older adults with and without cognitive impairments. Understanding the mediative effect between acute exercise and increased performance is critical towards utilizing exercise training as a tool for improvement of brain structure and health.Acknowledgements
F31-HD103361, R01-AG058853, Delaware INBREReferences
1. Chang YK, Labban JD, Gapin JI, Etnier JL. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012;1453(250):87-101. doi:10.1016/j.brainres.2012.02.068
2. Manduca A, Oliphant TE, Dresner MA, et al. Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Med Image Anal. 2001;5(4):237-254. doi:10.1016/S1361-8415(00)00039-6
3. Hiscox L V, Johnson CL, Barnhill E, et al. Magnetic resonance elastography (MRE) of the human brain : technique , findings and clinical applications. Phys Med Biol. 2016;61:R401-R437. doi:10.1088/0031-9155/61/24/R401
4. Sandroff BM, Johnson CL, Motl RW. Exercise training effects on memory and hippocampal viscoelasticity in multiple sclerosis: a novel application of magnetic resonance elastography. Neuroradiology. 2017;59(1):61-67. doi:10.1007/s00234-016-1767-x
5. McIlvain G, Cerjanic AM, Christodoulou AG, McGarry MD, Johnson CL. OSCILLATE: A Low-Rank Approach for Accelerated Magnetic Resonance Elastography. In: 28th Annual Meeting of the International Society for Magnetic Resonance in Medicine. Abstract 0169; 2020.
6. Johnson CL, Holtrop JL, McGarry MDJ, et al. 3D multislab, multishot acquisition for fast, whole-brain MR elastography with high signal-to-noise efficiency. Magn Reson Med. 2014;71(2):477-485. doi:10.1002/mrm.25065
7. McGarry MDJ, Houten EEW Van, Johnson CL, et al. Multiresolution MR elastography using nonlinear inversion. Med Phys. 2012;39(10):6388-6396. doi:10.1118/1.4754649
8. McGarry MDJ, Van Houten EEW. Use of a Rayleigh damping model in elastography. Med Biol Eng Comput. 2008;46(8):759-766. doi:10.1007/s11517-008-0356-5
9. Murphy MC, Huston III J, Ehman RL, Huston J, Ehman RL. MR elastography of the brain and its application in neurological diseases. Neuroimage. 2019;187(August 2017):176-183. doi:10.1016/j.neuroimage.2017.10.008
10. Tarumi T, Zhang R. Cerebral hemodynamics of the aging brain: Risk of Alzheimer disease and benefit of aerobic exercise. Front Physiol. 2014;5 JAN(January):1-6. doi:10.3389/fphys.2014.00006
11. Barnes JN. Exercise, cognitive function, and aging. Adv Physiol Educ. 2015;39(1):55-62. doi:10.1152/advan.00101.2014
Figures