0106
Improved Quantification of Ktrans with Cardiac Output Based Correction of Arterial Input Function1Department of Radiology, New York University School of Medicine, New York, NY, United States, 2School of Biomedical Engineering, ShanghaiTech University, Shanghai, China, 3Department of Radiology, Weill Cornell Medical College, New York, NY, United States
Synopsis
The arterial signal in DCE MRI often suffers from the inflow effect, which may cause large errors in the arterial concentration and compartmental model analysis. We implemented a constrained signal-to-concentration conversion using the subject’s cardiac output based on the Stewart-Hamilton principle, which limits the area under the arterial first pass peak. The constrained conversion significantly reduced the variation of the arterial concentration sampled at three levels along the abdominal aorta. The constrained arterial input function resulted in a significantly lower variability of the Tofts model Ktrans in psoas muscle compared to the uncorrected input function.
Introduction
Reliable estimates of the arterial input function (AIF) are critical to the model analyses of dynamic contrast-enhanced (DCE) MRI. However, the blood signal is often affected by various factors, such as the RF coil sensitivity, hematocrit, and inflow artifact, which may cause large errors in model parameters1. We implemented a method to compute the AIF concentration constrained by the subject’s cardiac output (CO). The method forces the area under the AIF’s first pass to obey the Stewart-Hamilton principle of indicator dilution2. Here we evaluate the effectiveness of the constrained conversion to reduce the variability of Ktrans due to the variations of the AIF sampled at different levels along the abdominal aorta.Methods
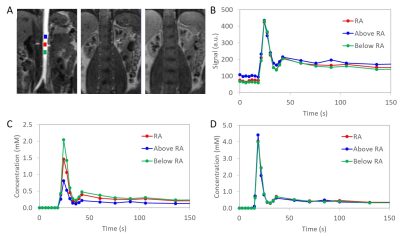
This retrospective study was approved by the institutional review board. Subjects were selected from an existing dataset of renal DCE MRI exams (N=116) acquired with the same protocol3. One female and one male subject were selected to represent each of 7 decades by age (20-29 years old, 30-39,…, 80-89 years old) for a total of 14 exams (7 women and 7 men; age, mean±stdev, 55±21 years; age range, 24-87 years). DCE MRI was acquired at 1.5 T (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Germany) for at least 6 min after an injection of 4 mL Gd-DTPA using 3D FLASH (TR=2.84 ms; TE=1.05 ms; FA=12°; matrix, 256x256x40; voxel, 1.66x1.66x2.5 mm3; temporal resolution, 3 s during 0-40 s post-injection and 15-60 s afterwards).Image analysis was performed in FireVoxel, a freely available research software package (NYU School of Medicine, firevoxel.org). Using fully automatic image derived input function (IDIF)4, we generated, for each subject’s abdominal aorta, three signal curves Si,j(t), where i=1,…,14 is the subject number and j=1,2,3 is the sampling location: (1) 3 cm superior to the renal arteries (RA), (2) at RA; (3) 3 cm inferior to RA.
Constrained signal-to-concentration conversion was implemented in FireVoxel (Fig. 1). The bolus arrival time (BAT) and recirculation time (RCT, the transition between the first pass peak and the recirculation peak) were identified automatically. These time points were used to find the baseline signal S0i,j and the area under the first pass peak, which was determined from the gamma variate fit5. The aortic signal curves Si,j(t) were first converted to concentration Ci,j(t) using regular conversion based on spoiled gradient echo signal equation and fast water exchange limit6 with T10=1480 ms for blood7 and contrast agent relaxivity8 r1=4.25 (mM s)-1. For each subject, the cardiac output (COi) was estimated as COi = CI x BSAi, where the cardiac index CI was assumed to be fixed at 2.7 L/min/m2 for all subjects9 and BSAi was the subject’s body surface area10. The CO-constrained concentration curves C’i,j(t) were computed by iterative fitting.
Regions of interest (ROIs) (24.2±12.1 cm3) were drawn over each subject’s psoas muscle. The ROI-averaged signal was converted to concentration with T10=1100 ms for muscle11. Muscle concentration was fitted with the two-parameter Tofts model12. Model fitting was performed with the original, unconstrained Ci,j(t) and then with constrained C’i,j(t) as the AIF, yielding Ktransi,j and Ktrans’i,j, respectively. The variation of Ktrans due to the AIF sampling level was estimated as Di = (|Ktransi,1 – Ktransi,2| + |Ktransi,2 – Ktransi,3| + |Ktransi,3 – Ktransi,1|)/3 and similarly as D’i with Ktrans’i,j.
The AIF variability due to sampling level was estimated as Ei = (P’i – Pi)/Pi, where Pi = (‖Ci,1(t) – Ci,2(t)‖ + ‖Ci,2(t) – Ci,3(t)‖ + ‖Ci,3(t) – Ci,1(t)‖)/Ci,avg and Ci,avg was each subject’s mean arterial concentration averaged across the three levels (Ci,1(t), Ci,2(t), Ci,3(t)) between BAT and the last time point. The post-correction P’ was defined similarly, with C’i,j replacing Ci,j. The correlation of the AIF difference with age was assessed using Pearson coefficient. The comparison of Pi and Pi’, Ktransi and Ktransi’ and Di and D’i, was done using one-tailed, paired t-test, assuming p=0.05 to indicate significance.
Results
The mean Ktrans was 0.107±0.090 mL/min/mL with regular conversion and 0.045±0.027 mL/min/mL with constrained conversion (p=0.005) (Fig. 2A). The variation of Ktrans was significantly reduced after constrained conversion (p=0.006) (Fig. 2B). The estimated CO (mean±stdev) was 5.4±0.7 L/min (women, 5.1±0.5 L/min; men, 5.7±0.8 L/min). The mean AIF differences decreased by 57%±20% (p<0.001) after constrained conversion (Fig. 3). The AIF variations tended to be larger in younger subjects with regular conversion (p=0.06), but this trend disappeared after constrained conversion. The differences of the aortic signal baseline with sampling level, which led to large variations of the concentration curves obtained with regular conversion, decreased after constrained conversion (Fig. 4).Discussion
The constrained conversion reduced both the mean values and the differences in Ktrans due to the variations of the AIF with the sampling location. The post-correction Ktrans values were in agreement with the values obtained in psoas muscle13 and in leg muscles14 using commercial software employing a population-based AIF. Here, we determined Ktrans using the subjects' own AIFs and individualized CO estimates. The resulting distribution of CO was in good agreement with published values15. The correction was less effective in a case with motion artifacts (Fig. 2B). The constrained conversion is expected to improve Ktrans quantification in well-perfused tissues, as well as in younger subjects with stronger inflow effect.Acknowledgements
NIH/NIBIB U24 EB02898References
1. Roberts C, Little R, Watson Y, Zhao S, Buckley DL, Parker GJ. The effect of blood inflow and B(1)-field inhomogeneity on measurement of the arterial input function in axial 3D spoiled gradient echo dynamic contrast-enhanced MRI. Magn Reson Med. 2011 Jan;65(1):108-19. PMID: 20928889.
2. Zhang JL, Rusinek H, Bokacheva L, Chen Q, Storey P, Lee VS. Use of cardiac output to improve measurement of input function in quantitative dynamic contrast-enhanced MRI. J Magn Reson Imaging. 2009 Sep;30(3):656-65. PMID: 19711414.
3. Lee VS. MR Angioplasty and Renography for Renovascular Disease. NIH/NIDDK R01 DK063183. 2003-2010. NYU School of Medicine.
4. Mikheev A, Logan J, Ding Y-S, Rusinek H. A novel two-stage iterative vessel tracking algorithm for determining an image derived input function for PET. J Nucl Med. May 2016; 57(Suppl 2), 1905.
5. Zhang X, Petersen ET, Ghariq E, De Vis JB, Webb AG, Teeuwisse WM, Hendrikse J, van Osch MJ. In vivo blood T(1) measurements at 1.5 T, 3 T, and 7 T. Magn Reson Med. 2013 Oct;70(4):1082-6. PMID: 23172845.
6. Shen Y, Goerner FL, Snyder C, Morelli JN, Hao D, Hu D, Li X, Runge VM. T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 T. Invest Radiol. 2015 May;50(5):330-8. PMID: 25658049.
7. Wolsk E, Bakkestrøm R, Thomsen JH, Balling L, Andersen MJ, Dahl JS, Hassager C, Møller JE, Gustafsson F. The influence of age on hemodynamic parameters during rest and exercise in healthy individuals. JACC Heart Fail. 2017 May;5(5):337-346. PMID: 28017352.
8. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Nutrition. Sep-Oct 1989;5(5):303-11. PMID 2520314.
9. Davenport R. The derivation of the gamma-variate relationship for tracer dilution curves. J Nucl Med 1983 Oct;24(10):945-8. PMID: 6352876.
10. Wake N, Chandarana H, Rusinek H, Fujimoto K, Moy L, Sodickson DK, Kim SG. Accuracy and precision of quantitative DCE-MRI parameters: How should one estimate contrast concentration? Magn Reson Imaging. 2018 Oct;52:16-23. PMID: 29777820; PMCID: PMC6102067.
11. Gold GE, Han E, Stainsby J, Wright G, Brittain J, Beaulieu C. Musculoskeletal MRI at 3.0 T: relaxation times and image contrast. AJR Am J Roentgenol. 2004 Aug;183(2):343-51. PMID: 15269023.
12. Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, Port RE, Taylor J, Weisskoff RM. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusible tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999 Sep;10(3):223-32. PMID: 10508281.
13. Heye T, Boll DT, Reiner CS, Bashir MR, Dale BM, Merkle EM. Impact of precontrast T10 relaxation times on dynamic contrast-enhanced MRI pharmacokinetic parameters: T10 mapping versus a fixed T10 reference value. J Magn Reson Imaging. 2014 May;39(5):1136-45. PMID: 25006630.
14. Rusinaru D, Bohbot Y, Djelaili F, Delpierre Q, Altes A, Serbout S, Kubala M, Maréchaux S, Tribouilloy C. Normative reference values of cardiac output by pulsed-wave Doppler echocardiography in adults. Am J Cardiol. 2021 Feb 1;140:128-133. PMID: 33144167.
15. Yoon MA, Hong SJ, Ku MC, Kang CH, Ahn KS, Kim BH. Multiparametric MR imaging of age-related changes in healthy thigh muscles. Radiology. 2018 Apr;287(1):235-246. PMID: 29239712.
Figures
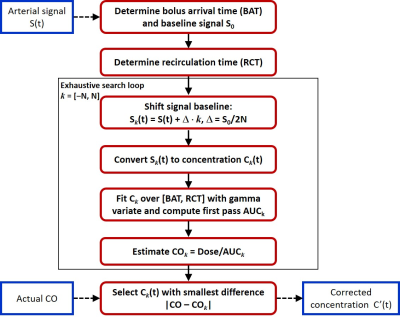
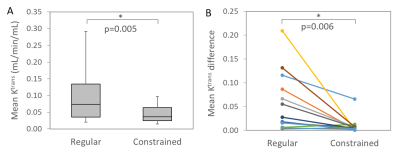
Figure 2: A. The mean Ktrans values in each subject due to the AIF variation with sampling level decreased significantly after constrained conversion (regular, 0.107±0.090 mL/min/mL; constrained, 0.045±0.027 mL/min/mL, p=0.005). B: The mean difference in each subject among Ktrans (mL/min/mL) due to the AIF variation with sampling level decreased significantly after constrained conversion (regular, 0.055±0.061; constrained, 0.009±0.017; p=0.006).
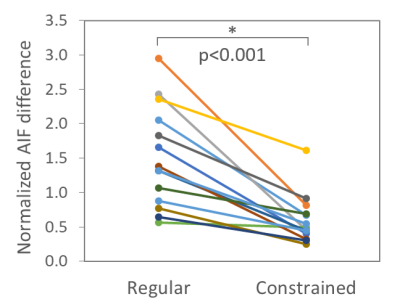
Figure 3: Normalized AIF difference over sampling levels in each subject decreased significantly after constrained conversion (mean±stdev: regular, 1.51±0.73; constrained, 0.60±0.35; p<0.001).