0104
Advances in accelerated imaging at 9.4T with electronically modulated time-varying receive sensitivities1Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 2Department of Biomedical Magnetic Resonance, Eberhard Karls University Tübingen, Tübingen, Germany
Synopsis
Previously, we have shown in simulations that electronically modulated time-varying receive sensitivities can improve parallel imaging reconstruction when fast modulations are applied during acquisition of k-space lines. Here, we demonstrate this concept experimentally with a prototype 8-channel reconfigurable receive coil, for which B1- modulation is achieved by fast switching PIN diodes in the receive loops. With this setup, MR measurements were performed in both phantom and human subject. Lower reconstruction errors and g-factors (~25% improvement for R=4) were observed for the case of rapidly switched sensitivities compared to conventional reconstruction with static sensitivities.
Introduction
In preceding work, we have demonstrated how time-varying receive sensitivities can improve parallel imaging (PI) performance1. For that, varactor diodes were used that continuously modulated capacitances in the receive loops and thus B1-. It was found that fast switching between the two extreme states of the varactors during frequency encoding of k-space lines yielded strongest PI improvement. To achieve the required switching speed experimentally, we demonstrate here a novel 8ch reconfigurable receive coil, for which B1- modulation is realized by fast switching PIN diodes. With this setup, we investigate PI performance for in vivo measurements.Methods
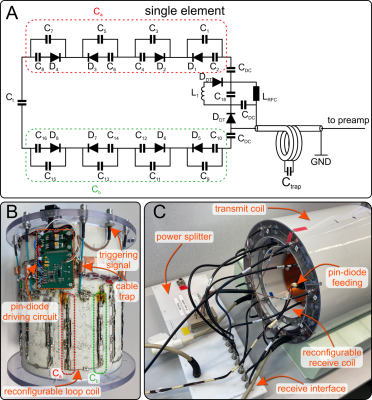
The prototypeThe proposed reconfigurable receive-only array consists of eight rectangular loops (20mm x 50mm), arranged symmetrically on a cylinder of 210mm diameter (Figure 1B). Two distinct receive sensitivity configurations (RSC) are formed by increasing the values of the distributed capacitors in one side of the loop and decreasing them in the other side, and vice versa. For that, we designed a switchable unit consisting of a PIN-diode, series capacitor of 20pF, and a small capacitor of 1.5pF…2.4pF connected in parallel (Figure 1A). When the PIN diodes are negatively biased in one of the arms (Ca or Cb), the effective capacitance of each switchable unit becomes ~2.5pF. At the same time, the PIN diodes in the second arm are shorted. Consequently, the effective capacitance of each switchable unit becomes ~22pF. When the switchable units in one arm are negatively biased, the PIN diodes in the second arm are shorted to tune the coil at the working frequency of 400MHz.
MR imaging
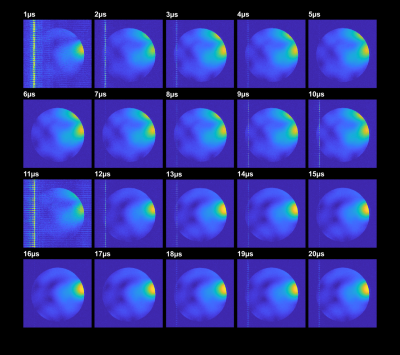
Measurements were performed on a 9.4T human whole body MR scanner (Siemens Healthineers, Erlangen, Germany) using a 2D multi-slice RF and gradient spoiled GRE sequence (TR/TE=20ms/8ms, FA=20°, matrix size 256x256, FOV=220mm x 220mm, slice thickness 5mm). Data were acquired in a homogeneous cylindrical phantom and a healthy subject after written informed consent and under approval of the local ethics committee. To capture RSC switching during k-space acquisition, 20-fold readout oversampling was applied for an ADC dwell time of 20µs, resulting in an effective dwell time of 1µs. Starting at the beginning of each ADC block, RSCs were switched every 10µs. From that, images of the RSC switching dynamics with a time resolution of 1µs could be obtained, which allows multiplexing information from both RSCs. Sensitivity maps for both RSCs were obtained via ESPIRIT2. Retrospective PI reconstructions and g-factor maps were calculated using SENSE3.
Results
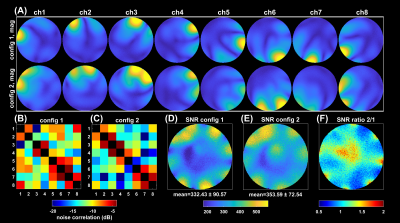
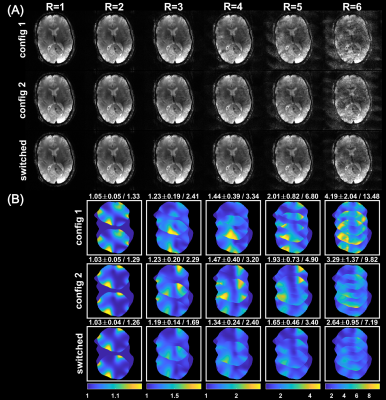
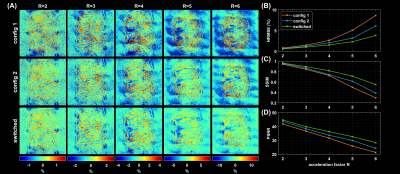
Switching the PIN diodes while the ADC is open caused spurious signal fluctuations of up to 8µs duration around the switching event, resulting in image artefacts (Figure 2). This can be corrected by excluding the corresponding samples from the reconstruction, which, however, reduces overall SNR. We are currently investigating possible hardware modifications to solve this issue.Fully sampled single coil images of each RSC are displayed in Figure 3A. The two reconfigurable coil states lead to distinct spatial sensitivity patterns, different noise correlations and spatial SNR distributions (Figure 3B-F). PI reconstruction results from retrospectively undersampled datasets of switched and static RSC measurements together with corresponding g-factor maps are given in Figure 4. Up to an acceleration factor of R=3, there is no visible difference between switched and static reconstructions, all showing low reconstruction errors. For R≥4, reconstructions from the static RSCs start to show increased noise amplification and artifacts outside of the brain, which is less pronounced in the switched case (Figures 4A, 5A). This is also reflected in the quality metrics in Figure 5B,C,D. Similarly, g-factors are lower for the switched case, e.g. for R=4: max g = 2.4 for switched compared to 3.34 and 3.20 for static RSCs, constituting an improvement of ~25%. The difference in g-factors between switched and static becomes more pronounced for higher acceleration factors.
Discussion
We showed that rapid receive sensitivity modulations can be realized by PIN diodes in the receive loops, which alter capacitances and thus B1-. Fast sensitivity modulation during acquisition of oversampled k-space lines yields the same g-factors as a hypothetical receiver array with double the number of channels such that the sensitivities of all RSCs are virtually active at the same time1. This observation corresponds to the time-division multiplexing SENSE reconstruction proposed for a rotating receive coil4. Additionally, fast RSC switching yields lower reconstruction artefacts, as more spatial encoding information is available than in case of static sensitivities. The observed improvements in PI performance compared to static RSCs are moderate. The main limitation is that sensitivity profiles of both RSCs are still similar and thus yield only partially independent spatial information. Consequently, future work will focus on new ideas to modify receive circuitry or geometry to achieve stronger B1- modulations.Conclusion
The reconfigurable receive coil array with fast switching PIN diodes allows rapid modulation of receive sensitivities during acquisition of k-space lines. This was demonstrated in vivo to improve PI performance, i.e. to yield lower g-factors (~25% improvement) and smaller reconstruction artefacts compared to static receive elements.Acknowledgements
We thank Alexander Loktyushin for insightful discussions. Financial support of the Max-Planck-Society, ERC Advanced Grant “SpreadMRI”, No 834940 and DFG Grant SCHE 658/12 is gratefully acknowledged.References
1. Glang F, Buckenmaier K, Bause J, Loktyushin A, Avdievich NI, Scheffler K. Investigations on accelerated imaging at 9.4T with electronically modulated time-varying receive sensitivities. Program Number 0910. In: Proc. Intl. Soc. Mag. Reson. Med. 29 (2021).
2. Uecker M, Lai P, Murphy MJ, et al. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magnetic Resonance in Medicine 2014;71:990–1001 doi: 10.1002/mrm.24751.
3. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magnetic Resonance in Medicine 1999;42:952–962 doi: 10.1002/(SICI)1522-2594(199911)42:5<952::AID-MRM16>3.0.CO;2-S.
4. Trakic A, Wang H, Weber E, et al. Image reconstructions with the rotating RF coil. Journal of Magnetic Resonance 2009;201:186–198 doi: 10.1016/j.jmr.2009.09.009.
Figures