0100
Turbo-spin echo based B1+ mapping in the presence of metallic hardware
Iman Khodarahmi1, Mahesh B Keerthivasan2, and Jan Fritz1
1Radiology, New York University School of Medicine, New York, NY, United States, 2Siemens Medical Solutions USA Inc, Malvern, PA, United States
1Radiology, New York University School of Medicine, New York, NY, United States, 2Siemens Medical Solutions USA Inc, Malvern, PA, United States
Synopsis
A robust B1+ mapping technique in the presence of metallic hardware is still unavailable. Our proposed B1+ quantification technique relies on turbo-spin echo or SEMAC acquisitions to decrease metal-related susceptibility artifacts while resolving B1+ values from signal variations at various sets of excitation and refocusing flip angles. Apriori knowledge of signal evolution is obtained by simulating the Bloch equations at each B1+ value. Phantom validation showed promising results, particularly at areas close to the metal surface, which are invisible with other mapping techniques.
Introduction
Inhomogeneity of the transmit B1+ field causes spatial variation of flip angles (FA). Mapping of such spatial distribution has applications in B1-shimming, quantitative MRI, and transmit coil quality control. The majority of available B1+ mapping techniques utilize gradient-echo imaging and, hence, will fail in the presence of metallic implants due to susceptibility-induced phase distortions. In addition, successful B1+ mapping near metallic implants requires the incorporation of advanced techniques such as slice encoding for metal artifact correction (SEMAC) (1) to reduce B0 artifacts. A previously proposed technique combined SEMAC and dual-angle methods (DAM) to estimate B1+ in the presence of metal (2); however, the contribution of stimulated echoes in a turbo-spin echo (TSE) acquisition, which is not accounted for with DAM, may negatively affect the outcomes. In this work, we present a new TSE-based method of B1+ quantification near metallic implants, which can be combined with SEMAC.Theory
B1+ field variations affect a TSE pulse sequence by proportionally scaling the excitation and refocusing (Ex-Ref) radiofrequency (RF) pulse flip angles. In the presence of a B1+ scale factor of b1 (b1 = actual B1+ / nominal B1+), the signal intensity can be expressed as: $$$S(b_1) = f(b_1.\theta(z), b_1.\phi(z))$$$, where θ(z) and φ(z) represent Ex-Ref RF profiles, respectively, and f(.) is the slice-resolved Bloch model. For different sets of Ex-Ref FA, b1 can be obtained by solving the following optimization problem: $$b_1 = arg min_{b_1} \sum_i^N||\int_{z} f(b_1.\theta_i(z), b_1.\phi_i(z))-I(\theta_i,\phi_i)||_2^2$$ with $$$I(\theta_i,\phi_i)$$$ being the pixel signal intensity obtained by the ith set of Ex-Ref FA.Methods
Simulations: The magnetization evolution for different Ex-Ref FA during TSE acquisitions was modeled by simulating the spatiotemporal propagation of spins according to Bloch equations (3). The exact pulse sequence scheme and parameters were used to simulate the signal evaluation while slice profiles were resolved by solving the Bloch equations. A database of the signal intensity for various sets of Ex-Ref FA and b1 was generated.Model validation: The model accuracy for different sets of Ex-Ref FA (n = 8) was tested on a clinical 3T MRI system (MAGNETOM Prisma; Siemens Healthcare GmbH, Erlangen, Germany) by placing a gel containing cylindrical tube at the iso-center of the magnet. Iso-center placement of the tube and its small diameter ensured B1+ homogeneity (b1 =1). To measure the magnetization evolution at each Ex-Ref FA, the phase-encoding gradients were switched off, and consequently, no spatial in-plane encoding was applied (4).
Hip arthroplasty MRI: Ceramic-on-polyethylene titanium (Ti) and metal-on-metal cobalt-chromium (CoCr) hip arthroplasty implants embedded in ASTM get medium (T1 = 1000ms, T2 = 60ms) were imaged with axial high transmit-receive bandwidth TSE and coronal SEMAC pulse sequences, respectively. Sequence parameters included TR/TE = 3000/24ms, voxel size = 0.5 x 0.5 x 3.0 mm3, and turbo factor = 13 for the Ti, and TR/TE = 1820/32ms, voxel size = 1.1 x 1.1 x 5.0 mm3, turbo factor = 13, and SEMAC steps = 16 for the CoCr implants. The signal intensities of these acquisitions were compared against the simulated database to solve the aforementioned minimization problem. For comparison purposes, b1 maps were also obtained using the method described in (5), which includes a TurboFLASH sequence equipped with a preceding RF pulse for magnetization preparation.
Results and Discussion
Simulated signal for various Ex-Ref FA sets as a function of b1 is shown in Figure 1. As seen, lower Ex-Ref FA sets (e.g. 30-60°) are more sensitive to higher b1 values, whereas higher sets (e.g. 120-180°) are more sensitive to lower b1. The Bloch model showed promising performance against the experimental data obtained with TSE sequence at b1 =1 (Figure 2), with a maximum of 8% signal underestimation observed at Ex-Ref FA of 30-60°. Axial images of the Ti implant along the femoral stem acquired with various Ex-ref FA sets and the estimated b1 map are shown in Figure 3. Higher b1 values were noted near the implant surface, in alignment with previously reported numerical simulations (6). Such high values were not detected with the TurboFLASH method due to dependencies between the preparation RF pulse and the b1 dynamic range in this method. A minimum of three Ex-Ref FA sets was capable of providing the b1 maps except for a thin rind at the metal surface. Figure 4 shows the b1 distribution in the coronal plane of the CoCr implant with the corresponding map acquired with the TurboFLASH technique. Due to high susceptibility artifacts of the CoCr, b1 quantification is only possible with the implementation of SEMAC.Conclusion
Our proposed technique estimates the spatial distribution of the B1+ field surrounding metallic implants by applying various excitation-refocusing schemes while using TSE or SEMAC acquisitions for metal artifact reduction. Promising results were obtained, particularly at areas close to the metal surface, which are invisible with other mapping techniques.Acknowledgements
No acknowledgement found.References
- Lu W, Pauly KB, Gold GE, Pauly JM, and Hargreaves BA. SEMAC: Slice Encoding for Metal Artifact Correction in MRI. Magn Reson Med. 2009;62:66-76.
- Monu UD, Worters PW, Sung K, Koch KM, Gold GE, and Hargreaves BA. B1 Mapping Near Metallic Implants. 19th annual meeting of the International Society for Magnetic Resonance in Medicine (ISMRM). Montre´ al, Que´bec, Canada. 2011:3171.
- Ben-Eliezer N, Sodickson DK, and Block KT. Rapid and Accurate T2 Mapping from Multi–Spin-Echo Data Using Bloch-Simulation-Based Reconstruction. Magn Reson Med. 2015;73:809-817.
- Liu H, van der Heide O, van den Berg CAT, and Sbrizzi A. Fast and accurate modeling of transient-state, gradient-spoiled sequences by recurrent neural networks. NMR in Biomedicine. 2021;34:e4527.
- Chung S, Kim D, Breton E, and Axel L. Rapid B1+ mapping using a preconditioning RF pulse with turboflash readout. Magn Reson Med. 2010;64(2):439-446.
- Bachschmidt TJ, Kohler M, Nistler J, Geppert C, Jakob PM, Nittka M. Polarized Multichannel Transmit MRI to Reduce Shading near Metal Implants. Magn Reson Med. 2016;75(1):217–226.
Figures
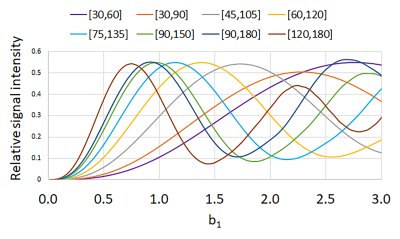
Figure 1. Bloch
simulated signal intensity as a function of b1 for various sets of excitation and
refocusing flip angles.
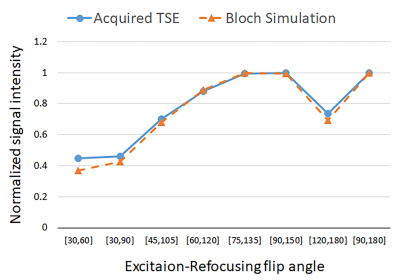
Figure 2.
Experimental and Bloch simulated signal intensity of various sets of excitation
and refocusing flip angles at b1 = 1.
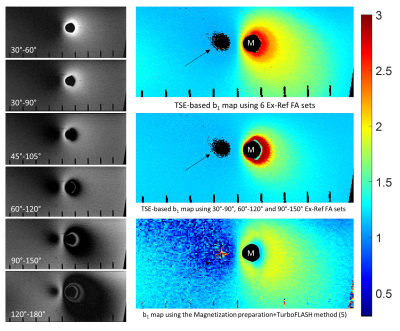
Figure 3. Left: High
transmit-receive bandwidth TSE images acquired at various Ex-Ref FAs. Right:
Estimated b1 maps by the proposed technique using 6 (top) and
3 (middle) Ex-Ref FA sets, and the TurboFLASH method (bottom) for comparison.
Arrows: area of shading where b1 cannot be estimated due to lack of signal. M: metal implant.
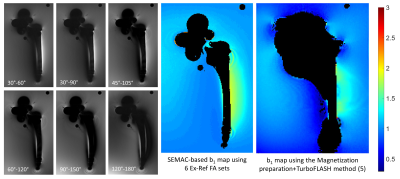
Figure 4. Left: SEMAC
images acquired at various Ex-Ref FAs. Middle: Estimated b1 map by the proposed technique using 6 Ex-Ref FA
sets. Right: Estimated b1 map using the TurboFLASH method for comparison. Black regions represent
the implant and associated susceptibility artifacts.
DOI: https://doi.org/10.58530/2022/0100