0099
The role of facet joint arthropathy in chronic low back pain and its association with adjacent paraspinal muscle composition1Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Department of Diagnostic and Interventional Radiology, University Hospital Ulm, Ulm, Germany, 3Department of Diagnostic and Interventional Neuroradiology, Technical University of Munich, Munich, Germany, 4Department of Orthopaedic Surgery, University of California San Francisco, San Francisco, CA, United States, 5Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, CA, United States
Synopsis
Low back pain (LBP) is a global health burden, but patient phenotyping based on imaging that would facilitate timely and effective treatment regimens lacks behind. One issue is that associations between different structures at the degenerative lumbar spine are not yet well characterized. In this study we revealed that facet joint arthropathy (FJA) at level L4/L5 is associated with the fat fraction (FF) of adjacent paraspinal musculature (PSM) as derived from chemical shift encoding-based water-fat MRI (CSE-MRI), as well as with Modic-type endplate changes, endplate defects, and intervertebral degenerative disk disease (DDD) in patients with chronic LBP.
Introduction
Globally, LBP shows a point prevalence of activity-limiting pain of about 7%, with >500 million people affected1,2. Notably, only in about 10% of patients can LBP be attributed to a specific pathology, while the great majority of patients is assigned a diagnosis of non-specific LBP3,4. This exerts negative impact on timely and efficient treatment initiation.One obstacle for better understanding of contributors to LBP is that many lumbar MRI findings are common in patients with and without LBP, thus hampering definition of causal relationships in individual patients5,6. Further, biomechanical properties of the lumbar spine and their alterations in response to improper load and degeneration seem key to advanced understanding of contributors to LBP, which would require not only focusing on one component, but investigating associations between different structures to elucidate pain mechanisms and patient phenotyping.
The lumbar facet joints (FJs) can be common generators of LBP7,8. They play important roles in load transmission, load-bearing, and restricting excessive axial rotation7,8. Concomitant DDD has been suggested a risk factor for FJA, but data are inconclusive and potential additional associations of FJA with other components that may relate to altered load sharing are scarce. A structure that is increasingly recognized as a relevant mediator in LBP is PSM9,10, which is adjacent to FJs and has recently been investigated by advanced quantitative imaging using CSE-MRI11,12. Against this background, this study’s hypothesis was that FJA is associated with the FF of PSM as well as with degenerative endplate, intervertebral disk, and vertebral body changes.
Methods
Eighty-four patients suffering from chronic LBP (36 females, mean age±SD: 44.6±13.4 years) were prospectively enrolled as part of the Back Pain Consortium (BACPAC) Research Program, a translational effort to address the need for effective and personalized therapies for chronic LBP. Patients with evidence of distinct patho-anatomical causes for LBP (e.g., disc herniation, spondylolisthesis, lumbar scoliosis, or spondylolysis) were excluded. All patients underwent 3-Tesla imaging of the lumbar spine, including sagittal and axial T1- and T2-weighted FSE and iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) sequences (Table 1)12,13. IDEAL processing considered phase error correction, a complex-based water-fat decomposition (pre-calibrated six-peak fat spectrum), and a single T2*14. The axial FF maps were computed as the ratio of the fat signal over the sum of fat and water signals15.A musculoskeletal radiologist (>30 years of experience) evaluated FJ changes using the grading by Pathria et al. (Figure 1)16, bone marrow lesions at the vertebral endplates (Modic-type endplate changes I-III)17, endplate defects (e.g., erosive intervertebral osteochondrosis or Scheuermann variant)18,19, and DDD using the Pfirrmann classification (Figure 2)20. The bilateral multifidus and erector spinae muscles were manually segmented21 (two consecutive slices per disc level spanning the L1-S1 levels; Figure 3), followed by extraction of the FF of PSM (from IDEAL; inter-rater agreement: intra-class correlation coefficient [ICC]=0.92; intra-rater agreement: ICC=0.98). Mixed effects models (adjusted for age, sex, and body mass index) were calculated to assess relationships between the different scorings, FF of PSM, and the Oswestry Disability Index (ODI)22.
Results
The FF of PSM was statistically significantly associated with the grading for FJA at level L4/L5 (β-coefficient: 1.77, 95%-confidence interval [95%-CI]: 0.57-2.96, p=0.004), but not at the other lumbar levels (p>0.05). Similarly, FJA was significantly associated with Modic-type endplate changes (β-coefficient: 0.13, 95%-CI: 0.04-0.22, p=0.006), endplate defects (β-coefficient: 0.11, 95%-CI: 0.03-0.19, p=0.011), and DDD (β-coefficient: 0.25, 95%-CI: 0.04-0.46, p=0.022) for level L4/L5, respectively.At level L4/L5, the mean FF±SD of PSM amounted to 17.8±9.1%. Table 2 provides an overview of the prevalence and grading of degenerative findings. Regarding self-reported pain and disability, FJA was statistically significantly associated with the ODI at levels L2/L3 (p=0.040), L4/L5 (p=0.005), and L5/S1 (p=0.008).
Discussion
The results of this study point towards an integrative model of degenerative spine pathology on the basis of FJA. By showing associations between FJA and Modic-type endplate changes, endplate defects, DDD, and, notably, FF of PSM, the interplay between different components at the degenerative spine is emphasized. Previous work using density measures from computed tomography (CT) or a muscle-fat index from conventional T1-weighted imaging suggested that fatty infiltration of PSM may relate to FJA23; yet, by means of CSE-MRI that provides valid quantitative measures of muscle composition24,25, this has not been demonstrated. Further, the herein revealed relationships amongst various spinal pathologies warrants future longitudinal studies to identify causal mechanisms and patient phenotypes most likely to benefit from pathology-specific treatments. Associations almost exclusively for the L4/L5 level seem to correspond to recent findings, demonstrating that cartilage endplate damage was predictive of symptoms when adjacent to PSM with high FF12. Yet, follow-up work–ideally combining imaging with finite element analysis to further explore the role of load sharing and FJA26–may reveal causes for the observed relationships at this specific level.Conclusion
In patients with chronic LBP, FJA is associated with a range of other degenerative changes at level L4/L5, supporting simultaneous investigation of multiple level-wise components to better explain pain and disability. Specifically, associations between various spinal pathologies motivates future longitudinal studies to elucidate causal mechanisms and to identify patient phenotypes that would most likely benefit from pathology-specific treatment strategies.Acknowledgements
This research was supported by the National Institutes of Health through the NIH HEAL Initiative under award numbers U19-AR076737 and UH2-AR076719 as well as by the German Academic Exchange Service (Deutscher Akademischer Austauschdienst, DAAD: N.S.), the Joachim Herz Foundation (N.S.), and the Rolf W. Günther Foundation (N.S.).References
- Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545-602.
- Vlaeyen JWS, Maher CG, Wiech K, et al. Low back pain. Nat Rev Dis Primers 2018;4:52.
- Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet 2017;389:736-47.
- Koes BW, van Tulder MW, Thomas S. Diagnosis and treatment of low back pain. Bmj 2006;332:1430-4.
- Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR American journal of neuroradiology 2015;36:811-6.
- Panagopoulos J, Magnussen JS, Hush J, et al. Prospective Comparison of Changes in Lumbar Spine MRI Findings over Time between Individuals with Acute Low Back Pain and Controls: An Exploratory Study. AJNR American journal of neuroradiology 2017;38:1826-32.
- Inoue N, Orias AAE, Segami K. Biomechanics of the Lumbar Facet Joint. Spine Surg Relat Res 2020;4:1-7.
- Mann SJ, Viswanath O, Singh P. Lumbar Facet Arthropathy. StatPearls. Treasure Island (FL)2021.
- Ranger TA, Cicuttini FM, Jensen TS, et al. Are the size and composition of the paraspinal muscles associated with low back pain? A systematic review. Spine J 2017;17:1729-48.
- Goubert D, Oosterwijck JV, Meeus M, Danneels L. Structural Changes of Lumbar Muscles in Non-specific Low Back Pain: A Systematic Review. Pain Physician 2016;19:E985-E1000.
- Sollmann N, Dieckmeyer M, Schlaeger S, et al. Associations Between Lumbar Vertebral Bone Marrow and Paraspinal Muscle Fat Compositions-An Investigation by Chemical Shift Encoding-Based Water-Fat MRI. Front Endocrinol (Lausanne) 2018;9:563.
- Bailey JF, Fields AJ, Ballatori A, et al. The Relationship Between Endplate Pathology and Patient-reported Symptoms for Chronic Low Back Pain Depends on Lumbar Paraspinal Muscle Quality. Spine 2019;44:1010-7.
- Reeder SB, Pineda AR, Wen Z, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2005;54:636-44.
- Boehm C, Diefenbach MN, Makowski MR, Karampinos DC. Improved body quantitative susceptibility mapping by using a variable-layer single-min-cut graph-cut for field-mapping. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2021;85:1697-712.
- Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. Journal of magnetic resonance imaging : JMRI 2012;36:1011-4.
- Pathria M, Sartoris DJ, Resnick D. Osteoarthritis of the facet joints: accuracy of oblique radiographic assessment. Radiology 1987;164:227-30.
- Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 1988;166:193-9.
- Jevtic V. Magnetic resonance imaging appearances of different discovertebral lesions. European radiology 2001;11:1123-35.
- Blumenthal SL, Roach J, Herring JA. Lumbar Scheuermann's. A clinical series and classification. Spine 1987;12:929-32.
- Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001;26:1873-8.
- Crawford RJ, Cornwall J, Abbott R, Elliott JM. Manually defining regions of interest when quantifying paravertebral muscles fatty infiltration from axial magnetic resonance imaging: a proposed method for the lumbar spine with anatomical cross-reference. BMC Musculoskelet Disord 2017;18:25.
- Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine 2000;25:2940-52; discussion 52.
- Cooley JR, Walker BF, E MA, Kjaer P, Jensen TS, Hebert JJ. Relationships between paraspinal muscle morphology and neurocompressive conditions of the lumbar spine: a systematic review with meta-analysis. BMC Musculoskelet Disord 2018;19:351.
- Fischer MA, Nanz D, Shimakawa A, et al. Quantification of muscle fat in patients with low back pain: comparison of multi-echo MR imaging with single-voxel MR spectroscopy. Radiology 2013;266:555-63.
- Guttsches AK, Rehmann R, Schreiner A, et al. Quantitative Muscle-MRI Correlates with Histopathology in Skeletal Muscle Biopsies. J Neuromuscul Dis 2021;8:669-78.
- Mengoni M. Biomechanical modelling of the facet joints: a review of methods and validation processes in finite element analysis. Biomech Model Mechanobiol 2021;20:389-401.
Figures
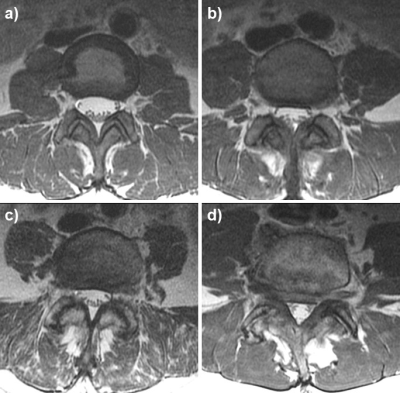
Figure 1: Facet joint (FJ) degenerative changes
Examples for grading of FJ changes: normal appearance (a); narrowing, small osteophytes or hypertrophy (b); narrowing, moderate osteophytes or hypertrophy (c); and narrowing, large osteophytes or severe hypertrophy (d).
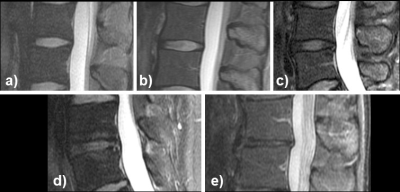
Figure 2: Degenerative disc disease (DDD)
Homogeneous, hyperintense, normal height of disc (a; Pfirrmann grade I); inhomogeneous, hyperintense, normal height, clear distinction annulus vs. nucleus (b; grade II); inhomogeneous, grey, normal height, no clear distinction between annulus vs. nucleus (c; grade III); inhomogeneous, grey to black, normal height to moderate loss, no distinction between annulus vs. nucleus (d; grade IV); and inhomogeneous, black, > 50% height loss (e; grade V).
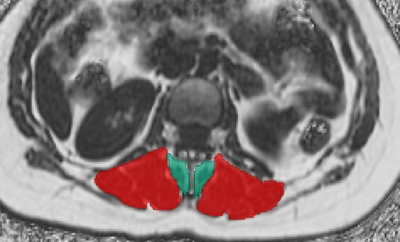
Figure 3: Segmentation of paraspinal musculature (PSM)
Segmentations of the multifidus muscles (green) and erector spinae muscles (red) of both sides. The segmentations of the multifidus and erector spinae muscles were used for extraction of the level-wise fat fraction (FF) of PSM (using IDEAL).
Table 2: Number of patients per grading score at level L4/L5
Facet joint arthropathy (FJA) was graded according to Pathria et al. (0–normal appearance to 3–narrowing, large osteophytes or severe hypertrophy), degenerative disk disease (DDD) according to Pfirrmann et al. (1–homogeneous, hyperintense, normal height of disc to 5–inhomogeneous, black, > 50% height loss), or present (1) versus absent (0) for bone marrow lesions at the vertebral endplates and endplate defects. XXX: respective score not present for specific grading.