0087
Evidence for Improved Left Atrial Blood Flow Dynamics after Pulmonary Vein Isolation in Patients with Atrial Fibrillation1Department of Radiology, Northwestern University, Chicago, IL, United States, 2Department of Radiology, University Hospital Basel, University of Basel, Basel, Switzerland, 3Department of Cardiology, Northwestern University, Chicago, IL, United States, 4Department of Preventive Medicine, Northwestern University, Chicago, IL, United States
Synopsis
Atrial fibrillation (AF) significantly increases stroke risk which is attributed to thrombus formation in the left atrium (LA) and to a greater extent the LA appendage (LAA). In this study, we investigated 4D-flow MRI-derived blood flow metrics from pre- and post-ablation exams in 26 AF patients undergoing pulmonary vein isolation (PVI). Blood stasis in both the LA and LAA significantly decreased in the post-PVI 4D-flow MRI. Furthermore, LA and LAA volumes also significantly decreased. Our results suggest that 4D-flow MRI was sensitive to detect treatment-related changes of LA and LAA flow metrics implicated on thromboembolic risk in AF patients.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia, and its incidence is expected to increase [1, 2]. The most serious complication of AF is increased stroke risk, attributed to thrombi originating in the left atrium (LA) and more frequently (90%) the left atrial appendage (LAA). There is strong evidence by echocardiography studies that changes in LA and LAA flow dynamics in AF (reduced flow velocities, increased stasis) are associated with thrombus formation and thus stroke risk [3-5]. Recent atrial 4D-flow MRI studies have shown that this technique can detect changes in LA hemodynamics in AF, i.e. potentially different predispositions to atrial thrombogenesis, that are not reflected in current clinical stroke risk models (like CHA2DS2-VASc score) [6-8]. Standard interventional treatment for AF is pulmonary vein isolation (PVI) to reduce AF burden [1, 9]. PVI is thought to reduce stroke risk, but studies directly investigating the impact of PVI on LA and LAA flow and stasis and thus individual risk for thromboembolism are lacking [10]. Therefore, we aimed to investigate intra-subject blood flow alterations in the LA and LAA derived from pre- and post-ablation atrial 4D-flow MRI exams.Methods
AF patients undergoing PVI between 09/2018 and 05/2021 were included, all gave written informed consent. PVI was performed according to standard protocol, post-ablation follow-up was extracted from the electronic patient records. Patients received two dedicated atrial MRI exams including 4D-flow MRI on 1.5T MRI systems (Siemens, Germany), the first prior to PVI, the second MRI minimum 3 months afterwards. 4D-flow MRI parameters: spatial resolution: 2.4x2.4x2.6mm, temporal resolution: 40ms, TE: 2.7ms, TR: 18.9ms, flip angle: 15°. After corrections for noise, eddy currents, and velocity aliasing, an experienced CV radiologist performed manual 3D-segmentations of the LA and LAA (Mimics, Materialize, Belgium), Figure 1. 3D-segmentations were used to mask the 4D-flow data and calculate median velocity (all voxels), peak velocity (top 5% of voxels) and stasis (percentage of voxels with blood flow <0.1cm/s) for both the LA and LAA [8]. To account for variable heart rates in AF, hemodynamic analysis was confined to systole, which was individually identified for each patient as the time from R-wave to mitral valve opening.Results
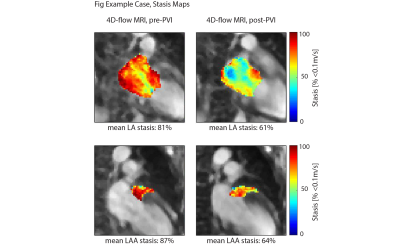
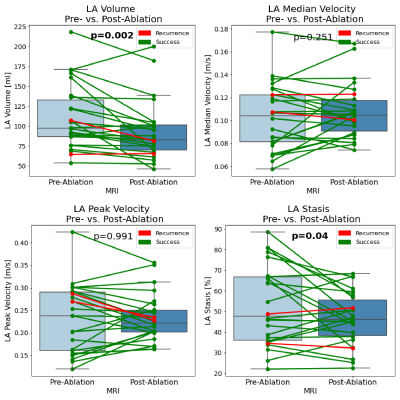
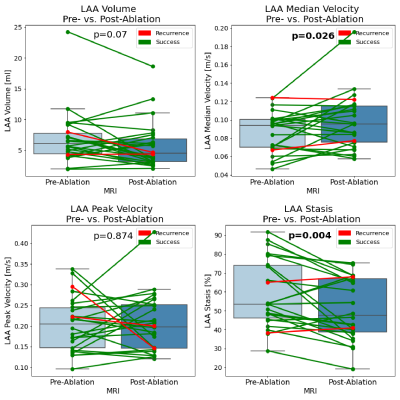
We included 26 patients (6 female, mean age 62±13years, mean BMI 26.7±4.3kg/m2). 23 patients had paroxysmal, 3 patients persistent AF. On average, there were 3.6±3.5days (median: 2days) between baseline MRI and PVI; 207±65days (median: 189.5days) were between PVI and follow-up MRI. Two patients suffered from AF recurrence and 23 maintained sinus rhythm (treatment success), mean follow-up time 324±167 days; one patient was lost to follow-up. As a representative example, Figure 2 shows an AF patient whose mean LA and LAA stasis decreased from 81% to 61% and from 87% to 64% pre- and post-ablation. Furthermore, LA volume decreased from 122ml to 105ml. As summarized in Table 1 and Figures 3 and 4, these findings were similar in the entire study cohort: LA volumes decreased significantly between pre- and post-ablation MRI (pre-PVI: 110.3±40.2ml vs. post-PVI: 92.7±36.6ml, p=0.002). In addition, there was a trend towards lower LAA volumes (pre-PVI: 6.9±4.3ml vs. 5.7±3.8ml, p=0.07). 4D-flow analysis revealed that PVI had a significant impact on LAA flow dynamics and resulted in reduced LAA stasis (pre-PVI: 58.9±17.8% vs. post-PVI: 50.8±16.0%, p=0.004; Figure 4) and increased median LAA velocities (pre-PVI: 0.09±0.02 m/s vs. post-PVI: 0.10±0.03 m/s, p<0.03). Additionally, LA stasis was significantly lower post-PVI (pre-PVI: 52.7±19.0% vs. post-PVI: 46.8±12.8%, p=0.04; Figure 3). Comparing pre- and post-PVI results for the two patients with AF recurrence, LA stasis was similar (pat-1: 34.6% vs. 32.3%, pat-2: 48.8% vs. 51.8%) and their LAA stasis even increased slightly (pat-1: 38.3% vs. 40.9%, pat-2: 65.1% vs. 68.0%).Discussion
Investigating pre- and post-PVI LA hemodynamics in AF patients demonstrated that the blood stasis, a measure of thrombogenic risk in AF, in the LAA and LA was significantly improved post-ablation. Moreover, we observed a significant decrease of LA and LAA volumes post-PVI. Overall, our result suggest a normalization of blood flow parameters in the LA and LAA post-ablation. LA volume decrease post-PVI is in good agreement with previous reports, for example a meta-analysis of 25 studies [11]. Comparing 4D-flow derived parameters to the literature, we observed pre-ablation LA stasis in the same range, whereas the LAA stasis post-PVI was lower in our cohort than reported in a small number of AF patients (n=6, no pre-ablation data available). LAA stasis in post-PVI patients was similar to the range reported for healthy controls [12]. This could indicate a marked reduction of risk for thrombus formation, especially in the LAA, as a result of PVI. Limitations of our study include incomplete coverage of the cardiac cycle by prospectively-gated atrial 4D-flow MRI and the use of static segmentations (common in 4D-flow analysis). Furthermore, the observed recurrence rate of 10% is lower than in the literature, likely related to varying follow-up length [13].Conclusion
Our study in AF patients demonstrated that atrial 4D-flow MRI was sensitive to detect significant post-ablation treatment changes of LA and LAA flow metrics implicated on thromboembolic risk. These findings indicate that 4D-flow MRI could be useful for assessment of PVI therapy as a means to reduce stroke risk in AF patients.Acknowledgements
No acknowledgement found.References
1. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Jr., Ellinor PT, Ezekowitz MD, Field ME, Furie KL et al: 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140(2):e125-e151.
2. Rahman F, Kwan GF, Benjamin EJ: Global epidemiology of atrial fibrillation. Nat Rev Cardiol 2014, 11(11):639-654.
3. Goldman ME, Pearce LA, Hart RG, Zabalgoitia M, Asinger RW, Safford R, Halperin JL: Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: I. Reduced flow velocity in the left atrial appendage (The Stroke Prevention in Atrial Fibrillation [SPAF-III] study). J Am Soc Echocardiogr 1999, 12(12):1080-1087.
4. Handke M, Harloff A, Hetzel A, Olschewski M, Bode C, Geibel A: Left atrial appendage flow velocity as a quantitative surrogate parameter for thromboembolic risk: determinants and relationship to spontaneous echocontrast and thrombus formation--a transesophageal echocardiographic study in 500 patients with cerebral ischemia. J Am Soc Echocardiogr 2005, 18(12):1366-1372.
5. Pollick C, Taylor D: Assessment of left atrial appendage function by transesophageal echocardiography. Implications for the development of thrombus. Circulation 1991, 84(1):223-231.
6. Lee DC, Markl M, Ng J, Carr M, Benefield B, Carr JC, Goldberger JJ: Three-dimensional left atrial blood flow characteristics in patients with atrial fibrillation assessed by 4D flow CMR. European heart journal cardiovascular Imaging 2016, 17(11):1259-1268.
7. Markl M, Lee DC, Furiasse N, Carr M, Foucar C, Ng J, Carr J, Goldberger JJ: Left Atrial and Left Atrial Appendage 4D Blood Flow Dynamics in Atrial Fibrillation. Circ Cardiovasc Imaging 2016, 9(9):e004984.
8. Markl M, Lee DC, Ng J, Carr M, Carr J, Goldberger JJ: Left Atrial 4-Dimensional Flow Magnetic Resonance Imaging: Stasis and Velocity Mapping in Patients With Atrial Fibrillation. Invest Radiol 2016, 51(3):147-154.
9. Shen MJ, Arora R, Jalife J: Atrial Myopathy. JACC Basic Transl Sci 2019, 4(5):640-654.
10. Barra S, Narayanan K, Boveda S, Primo J, Gonçalves H, Baran J, Agarwal S, Marijon E, Providência R: Atrial Fibrillation Ablation and Reduction of Stroke Events. Stroke 2019, 50(10):2970-2976.
11. Xiong B, Li D, Wang J, Gyawali L, Jing J, Su L: The Effect of Catheter Ablation on Left Atrial Size and Function for Patients with Atrial Fibrillation: An Updated Meta-Analysis. PloS one 2015, 10(7):e0129274-e0129274.
12. Fluckiger JU, Goldberger JJ, Lee DC, Ng J, Lee R, Goyal A, Markl M: Left atrial flow velocity distribution and flow coherence using four-dimensional FLOW MRI: a pilot study investigating the impact of age and Pre- and Postintervention atrial fibrillation on atrial hemodynamics. Journal of magnetic resonance imaging : JMRI 2013, 38(3):580-587.
13. Bavishi AA, Kaplan RM, Peigh G, Diaz CL, Baman JR, Trivedi A, Wasserlauf J, Shen MJ, Sattayaprasert P, Chicos AB et al: Patient characteristics as predictors of recurrence of atrial fibrillation following cryoballoon ablation. Pacing Clin Electrophysiol 2019, 42(6):694-704.
Figures