0084
Evaluation of operation pressure of a soft robotic extra-aortic cardiac assist device using MRI1Department of Radiology, Stanford University, Palo Alto, CA, United States, 2Cardiovascular Institute, Stanford University, Palo Alto, CA, United States, 3Department of Mechanical Engineering, Stanford University, Palo Alto, CA, United States, 4Department of Computer Science, Technical University of Munich, Garching, Germany, 5Institute for Medical Engineering & Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 6Department of Radiology, Stanfor University, Palo Alto, CA, United States, 7Division of Radiology, Veterans Affairs Health Care System, Palo Alto, CA, United States
Synopsis
MRI can be used to evaluate the interaction of soft robotic devices with biological tissues. The aim of this study was to use MRI to optimize the actuation pressure of an extra-aortic soft robotic cardiac assist device. We evaluated wall deformations, displaced fluid volumes and qualitative flows induced by the actuator on a fluid filled elastic vessel. Our results show that the displaced volume increases with the actuator pressure and decreases with increasing the vessel pressure. The maximum actuator pressures do not lead to buckling of the vessel wall given the current three-bladder design.
Background
Soft robots interact favorably with humans because the material properties can match those of soft biological tissue.1 Soft robots have been shown to mechanically assist the failing heart. While epicardial attachment avoids blood contact, securing the robot to the heart remains challenging.2 In contrast, soft robots are more easily wrapped around large blood vessels.3 We built an extra-aortic soft robot for hemodynamic support. Still, modeling and measuring the interaction of soft actuators with biological tissues remains a challenge, given their viscoelastic properties, curvilinear surfaces, and large deformations. We propose to use MR as an imaging modality to measure deformation, displaced volume and flows in mock circulatory flow loops to enable design optimization of a programmable soft robot. One actuator that suites for extravascular actuation is the McKibben pneumatic artificial muscle.4 Upon actuation the soft robot contracts, displaces volume, and in combination with the aortic valve creates forward flow. The McKibben actuator achieves a high contraction ratio when pressurized and can operate at a similar frequency to the heart.5 The aim of this work was to use MRI for assessing the pressure and volume displacement of a soft robotic McKibben actuator interacting with a compliant synthetic vessel.Methods
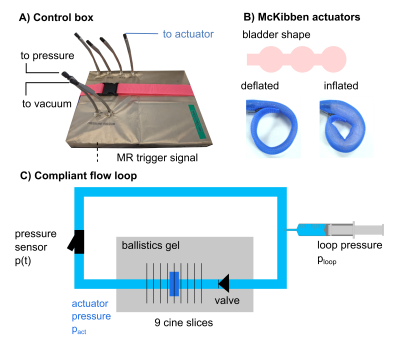
We built an MRI compatible electropneumatic control system and flow loop for testing a soft robot inside an MRI scanner. The control system2 pressurizes the actuator for 300 ms, then depressurizes for 700 ms cyclically (60 bpm) at 6, 8, 10, and 12psi and retrospectively gates image acquisition (Figure 1A). To address both MRI safety and potential radiofrequency (RF) artefacts, the design of the control system was Faraday-shielded (Cable Management, Electriduct, USA). One McKibben actuator was manufactured using a pre-fabricated mesh sleeve (Flexo PET, Techflex, USA) and a thermoformed inner bladder (Stretchlon 800, Fibreglast, USA). The three-bubble shape may minimize vessel wall deformation (Figure 1B). The actuator was wrapped around compliant tubing (high-temperature silicone rubber, durometer 35A) filled with a blood mimicking fluid (40% glycerol/60% water), surrounded by static gel, and was placed shortly downstream of a backflow valve (Figure 1C). The intra-vessel pressure was tuned to 50, 75, 100, and 150 mmHg. Pressure induced by the actuator was measured on the benchtop prior to MRI acquisition using a pressure transducer (SPR350-S, Millar) sampling at 1 kHz. We performed MR imaging using a clinical 3T MRI scanner (Skyra, Siemens). We used (1) fast low angle shot (FLASH) cine: 9 cross-sectional slices, TE=2.83ms, TR=29.6ms, Flip angle=12°, FOV=105x260 mm2, two averages, spatial resolution = 1.0x1.0x5.0mm, and retrospective gating with 40 temporal frames and (2) conventional 4D Flow sequence with Cartesian k-space sampling: VENC=25cm/s, TE=3.99ms, TR=77.28ms, Flip angle=15°, FOW=208x319mm2, spatial resolution=2.4x2.4x2.4mm, retrospective gating with 20 temporal frames. Cross-sectional areas of cine images were measured by an automated boundary detection method (MATLAB R2021a) and displaced volumes calculated by multiplying by slice thickness. Pressure data was down-sampled for pressure-volume loop analysis. Measurements of 4D flow were Eddy current corrected and visualized using Arterys (Arterys Inc., San Francisco, USA).Results
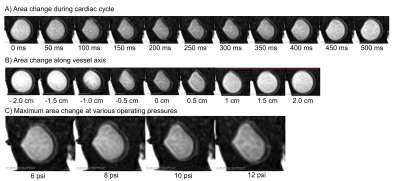
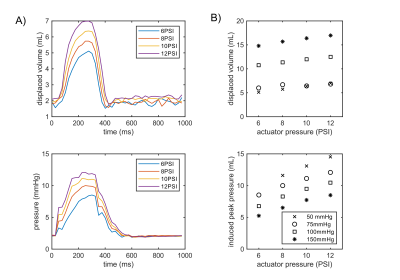
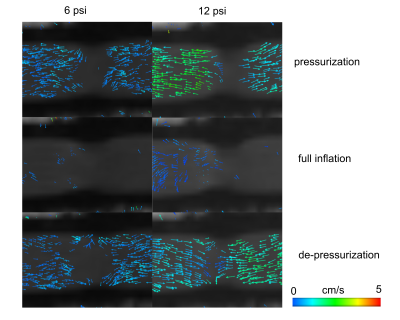
The vessel wall was deformed three-ways by each bubble of the actuator. The actuator is inflated for 300 ms, however the wall deformation persists until 400ms (Figure 2A). Observable wall deformations extend to +/- 1 cm from the actuator location (Figure 2B). The maximally induced wall deformation was dependent on both actuator and loop pressure. At high actuator pressures the vessel walls did not touch (Figure 2C). The timing of the incidence of the volume displacement is earlier with higher actuator pressures (Figure 3A). The maximally displaced volume (4.5–10 mL per beat) was positively related to the actuator pressure and negatively related to the loop pressure (Figure 3). The trends in timing were consistent with induced pressures measured on the benchtop. Combining the pressure and volume time-series data, the actuator was shown to produce positive stroke work. Qualitative measurements of 4D Flow indicate increased forward flow with increased operating pressure (Figure 4).Discussion
We designed an MRI-compatible control system with low ferromagnetic componentry, RF shielding, and one that did not produce any imaging artifacts. Traditional optical and benchtop methods are more limited in characterizing the interaction of soft robots with soft biological tissue. Particle image velocimetry offers an optical alternative, but does not capture flow and tissue motion simultaneously and is challenging upon large deformations.6 Here we show that MRI offers a comprehensive imaging-based evaluation of an extra-aortic cardiac assist device. The actuator pressure range tested shows that the current design does not cause the vessel walls to touch or buckle upon actuation. Larger actuator pressures displace more volume and may be preferred for more effective cardiac support. As too large actuator pressures can result in bladder failure, redesigning bladders with increased volume might help to further increase displaced volumes. Finally, the effect of actuator pressure on inflation timing may originate from the long pneumatic tubing used for remote actuation. In future work, we intend to use MRI to enable patient-specific design optimization of extra-vessel soft robots.Conclusion
Using MRI, we characterized the hemodynamic effects of an extra-aortic soft robot. Maximum actuator operating pressure does not lead to buckling of the vessel wall and induces forward flow.Acknowledgements
This study was supported by NIH R01 HL131823 to DBE. Swiss National Research Foundation P2EZP2_188964 to SAD.References
1. Roche ET, Wohlfarth R, Overvelde JTB, et al. A Bioinspired Soft Actuated Material. Adv Mater. 2014;26(8):1200-1206. doi:10.1002/adma.201304018
2. Roche ET, Horvath MA, Wamala I, et al. Soft robotic sleeve supports heart function. Sci Transl Med. 2017;9(373):eaaf3925. doi:10.1126/scitranslmed.aaf3925
3. Almanza M, Clavica F, Chavanne J, et al. Feasibility of a Dielectric Elastomer Augmented Aorta. Adv Sci. 2021;8(6):2001974. doi:10.1002/advs.202001974 4. Rus D, Tolley MT. Design, fabrication and control of soft robots. Nature. 2015;521(7553):467-475. doi:10.1038/nature14543
5. Chou C-PCC-P, Hannaford B. Static and dynamic characteristics of McKibben pneumatic artificial muscles. Proc 1994 IEEE Int Conf Robot Autom. 1994;(3):281-286. doi:10.1109/ROBOT.1994.350977
6. Medero R, Hoffman C, Roldán-Alzate A. Comparison of 4D Flow MRI and Particle Image Velocimetry Using an In Vitro Carotid Bifurcation Model. Ann Biomed Eng. 2018;46(12):2112-2122. doi:10.1007/s10439-018-02109-9
Figures